
Genotypic
and phenotypic variation of CADASIL among Chinese, Indians and Rungus in
Malaysia
Tsun-Haw Toh 1, Kheng-Seang Lim 1*,
Ching-Ching Ng 2, Imran Idris 1, Sherrini Bazir Ahmad 3,
Thien-Thien Lim 4, Irene Looi 5,
Ai-Huey Tan 1, Chung-Kin Chan 2, Chun-Shen Lim 6
and Chong-Tin Tan 1
1 Division of Neurology, Department of
Medicine, University of Malaya, Kuala Lumpur, Malaysia.
2 Genetics and Molecular Biology, Institute
of Biological Sciences, Faculty of Science, University of Malaya, Kuala Lumpur,
Malaysia.
3 Subang Jaya Medical Centre, Kuala Lumpur,
Malaysia.
4 Division of Neurology, Island Hospital,
Penang, Malaysia.
5 Clinical Research Centre, Seberang Jaya
Hospital, Penang, Malaysia.
6 Department of Biochemistry, School of
Biomedical Sciences, University of Otago, Dunedin, New Zealand.
* Correspondence: kslimum@gmail.com; Tel.: +603-79494477
Received: 10 June 2019; Accepted: 4 August 2019; Published: 17 August
2019; Corrected: 17 March
2020
Edited by: King-Hwa Ling (Harvard Medical School, Boston, USA)
Reviewed by: Azlina Ahmad Annuar (University of Malaya, Malaysia);
Chumpol Anamnart (Chulalongkorn University, Thailand)
Original article: https://doi.org/ 10.31117/neuroscirn.v2i3.35
Corrigendum: https://doi.org/ 10.31117/neuroscirn.v3i1.45
ABSTRACT: Cerebral autosomal dominant
arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is a
hereditary disease of small cerebral arteries. This case series aims to
describe the mutations in NOTCH3 and their phenotypes in Malaysia. We
included patients who were genetically confirmed to have CADASIL, diagnosed at the
University of Malaya Medical Centre, Malaysia. Family members who fulfilled
clinical or imaging criteria, and patients from two previous published
Malaysian families were also included. Six families (eleven cases) were
included in this series. Genetic testing revealed NOTCH3 mutations in
c.328C>T (p.Arg110Cys, R110C), c.553T>G (p.Cys185Gly, C185G),
c.1630C>T (p.Arg544Cys, R544C) and c.160C>T (p.Arg54Cys, R54C). Two out of
four Chinese families had R544C mutation in exon 11, with a later age of onset,
absence of migraine and lack of anterior temporal pole involvement on MRI. One
family with mixed Indian and Chinese ancestry had a mutation in exon 3 with
R110C and another Indian family exon 4 with C185G mutation. This case series
highlights the genotypic and phenotypic variability of CADASIL in a multiethnic
country. The finding of p.Arg544Cys mutation among the older Chinese families,
similar to those reported in Jeju Island and Taiwan, suggest the need to screen
the older Chinese stroke patients with typical MRI changes.
Keywords: CADASIL; Malaysia; R544C
©2019 by Toh et al. for use and distribution in accord with the
Creative Commons Attribution (CC BY-NC 4.0) license (https://creativecommons.org/licenses/by-nc/4.0/),
which permits unrestricted non-commercial use, distribution, and reproduction
in any medium, provided the original author and source are credited.
1.0 INTRODUCTION
Cerebral autosomal dominant arteriopathy with
subcortical infarcts and leukoencephalopathy (CADASIL) is a hereditary disease
of small cerebral arteries that has been recognised as an important cause of
stroke in the younger age group. Clinically, CADASIL is characterised by
transient ischaemic attacks (TIA) or ischaemic stroke, migraine with aura,
neuropsychiatric symptoms such as depression or bipolar disorder, as well as
dementia. Typical neuroimaging features include T2-hyperintensities in the
periventricular white matter, anterior temporal pole, external capsule, basal
ganglia, and brainstem [1]. Mutations in the NOTCH3 (notch receptor
3) gene on chromosome 19p13.2 are responsible for the clinical features in CADASIL, and up to date, more
than 270 different NOTCH3 mutations have been identified in CADASIL patients [2].
The number of CADASIL cases reported in Asia is
increasing [2-8] with better recognition of the condition and improved
access to genetic testing. Few studies have compared the mutation profiles
between Asians and Caucasians. Liu et al. [6] found that the mutational spectrum of NOTCH3
in northern China in all confirmed CADASIL patients was mostly in exon 4 and
exon 3, similar to Caucasians. However, other studies such as those in eastern
and southern China, Taiwan and Korea showed a different result, with the
majority being mutations in exon 11 [2-4]. The neuroimaging features in these studies were also
different, with a lower percentage (20-45.8%) of anterior temporal pole
involvement and a higher prevalence of intracranial haemorrhage [3,4,7]
Previous reported genetically confirmed cases from
Malaysia include a Rungus family [8] and a Chinese Malaysian family who resides
in Singapore [5]. In concordance with the multiethnic
diversity of the country, this case series highlights the variations in
genotypes and phenotypes among various ethnic groups in Malaysia.
2.0 METHODS
Ethics
approval was obtained from the ethics committee at the University Malaya
Medical Centre (UMMC). All subjects were Malaysian adults who presented to UMMC
and fulfilled our screening criteria as shown in Table 1. The subjects must
have at least one of the following: 1) stroke-like episodes with permanent
neurological signs, 2) migraine, 3) major mood disorder or subcortical
dementia. White matter changes in the brain MRI without cortical infarcts is
also a requirement, and there should be no vascular risk factors etiologically
related to the deficits. We excluded patients who had severe hypertension or
complicated heart/ vascular disease, those with severe intracranial stenosis on
magnetic resonance angiography (MRA)/ transcranial
Doppler (TCD) and those with normal brain magnetic resonance imaging (MRI) at
age > 35 years old. Vascular risk factors included
severe hypertension or diabetes mellitus. Severe hypertension is defined as
hypertension with target organ damage, including left ventricular hypertrophy,
heart failure, coronary artery disease, peripheral vascular disease, proteinuria,
glomerular filtration rate (GFR) < 60ml/min/1.73m2, papilloedema
or haemorrhage and exudates on retinal examination. Complicated heart/vascular
disease includes infective endocarditis, congenital heart disease, septal
defect, severe heart failure, left ventricular clot, aortic dissection or
carotid artery stenosis > 70%.
A total of six patients who fulfilled the criteria
above were identified at Neurology Unit, University of Malaya Medical Centre
from 2011 until 2017. Out of the six who underwent genetic testing, four were
tested positive for CADASIL NOTCH3 mutation. Family members who had features of either a young-onset
TIA or ischaemic stroke, migraine with aura, neuropsychiatric symptoms such as
depression or bipolar disorder, or dementia, were also included.
Table 1. Screening
criteria for CADASIL in this study
|
Inclusion criteria
|
|
1)
At least one of the following -
*
Stroke like episodes with permanent neurological signs;
*
Migraine;
*
Major mood disorder;
*
Subcortical dementia.
2)
No vascular risk factors
etiologically related to the deficits
3)
Abnormal MRI imaging of the
white matter without cortical infarcts
4)
Any age of onset.
|
|
Exclusion criteria
|
|
1)
Severe hypertension or
complicated heart or vascular disease
2)
Severe intracranial stenosis on
MRA or TCD
3)
Normal MRI imaging at age >
35.
|
A
detailed clinical assessment of the proband and their family members was
performed, especially for the presence of stroke, migraine, cognitive
dysfunction and psychiatric disorder, using semi-structured interview and
structured questionnaires (Supplement Form 1). In addition, new presentations
that were not described before were explored. A detailed pedigree was plotted.
Cases from the same family were denoted with the same number (such as 3 and 3.1
annotated the proband and her family member respectively). Investigations to
exclude other causes of stroke such as electrocardiogram, echocardiography,
lipid profile and diabetes screening were also performed.
To
have a more complete profile of a Malaysian case series, two previously
reported Malaysian families were included in the analysis, consisting of a
Rungus family reported by Lim et al. [8] and a Malaysian Chinese family by Wilder-Smith et
al. [5].
2.1
Neuroimaging study
The
cases underwent MRI of the brain using a 1.5T machine. The MRI sequences utilised
were Axial T2W, T1W, fluid attenuation inversion recovery (FLAIR) and MRA. The
lesions seen on MRI were quantified using Scheltens scoring system [9].
2.2 Blood samples
collection, DNA extraction and mutations screening
About
3 ml of peripheral blood was collected from each proband. Genomic DNA was extracted
using the QIAamp DNA Blood Midi kit (Qiagen, Hilden, Germany) according to
the manufacturer's instructions. Twenty-three exons of NOTCH3 of
the proband were amplified by PCR using Q5 hot start high-fidelity DNA
polymerase (NEB, Ipswich, MA, USA) or Phusion Flash high-fidelity PCR
master mix (Finnzymes, Thermo Scientific, Lafayette, CA, USA). The PCR products
were purified, followed by DNA sequencing. The sequencing results were
compared with NOTCH3 RefSeqGene (NM_000435.3).
Only those with pathogenic mutations in the coding regions of NOTCH3
were included. Mutations leading to the gain or the loss of a cysteine residue
in one of the 34 epidermal growth factor-like repeat (EGFr) domains of the
NOTCH3 protein (amino acid residues 40-1373)
are likely to be pathogenic [10] .
3.0 RESULTS
The
demographic and clinical features of the cases are shown in Table 2. Four out
of five cases with pure Chinese ethnicity (cases 1, 2, 6, 6.2) were found to
have an older age at diagnosis (ranging from 60 to 69 years) and did not have
any symptoms of migraine. They were screened for CADASIL due to the presence of
stroke and excessive white matter MRI changes without significant vascular
risk factors. Two cases had seizures (case 1 and 3), a rare but previously
reported symptom in CADASIL. We were also able to uncover some atypical
clinical features, including the presence of retinitis pigmentosa in case 3 and
a presentation of cerebellar stroke in case 6. The family pedigrees for cases
with a positive family history are shown in Figure 1. All cases demonstrated the
positive family history, except case 2.
Table
2. Demographics, clinical features
and genotypes of the CADASIL cases
|
Study
|
Our study
|
Lim et al. (2015)
|
Wilder-Smith et al. (2004)
|
|
Case
|
1
|
2
|
3
|
3.1
|
4
|
5
|
5.1
|
5.2
|
6
|
6.1
|
6.2
|
|
Age (onset)
|
68
|
60
|
30
|
20's
|
26
|
42
|
38
|
Asymptomatic
|
69
|
45
|
60
|
|
Gender
|
Male
|
Female
|
Female
|
Male
|
Male
|
Male
|
Male
|
Female
|
Female
|
Female
|
Male
|
|
Ethnicity
|
Chinese
|
Chinese
|
Mixed Chinese and Indian
|
Mixed Chinese and Indian
|
Indian
|
Rungus
|
Rungus
|
Rungus
|
Chinese
|
Chinese
|
Chinese
|
|
Family history*
|
Father and brother - C
|
Nil
|
Grandmother, Uncle, Aunty, Mother, 2nd
brother - C
Eldest brother - B
|
|
Grandparents, Parents, Uncle, 2 cousins -
C
|
Brother - A
Sister - A (asymptomatic at 45)
Brother - C
Mother - C
|
|
|
Brother - B
Niece - A
Father, 3 brothers,
2 sisters - C
|
|
|
|
Hypertension
|
Yes
|
No
|
No
|
No
|
No
|
No
|
No
|
No
|
Yes
|
No
|
No
|
|
Diabetes
|
No
|
No
|
No
|
No
|
No
|
No
|
No
|
No
|
No
|
No
|
No
|
|
Dyslipidaemia
|
No
|
No
|
Yes
|
No
|
Yes
|
Yes
|
No
|
No
|
Yes
|
No
|
No
|
|
Smoking
|
No
|
No
|
No
|
No
|
No
|
No
|
No
|
No
|
No
|
No
|
Yes
|
|
Initial symptom
|
Retrograde amnesia, Stroke
|
Stroke
|
Migraine with aura
|
Migraine with aura
|
Stroke
|
Migraine with aura
|
Stroke
|
Nil
|
Stroke (cerebellar)
|
Stroke
|
Stroke
|
|
Other manifestations
|
Seizure
|
Nil
|
Encephalopathy, Retinitis pigmentosa,
Stroke, Seizures
|
Stroke, Cognitive impairment
|
Migraine, Encephalopathy
|
Nil
|
Gelastic dementia
|
Nil
|
Nil
|
Cognitive impairment
|
Dementia
|
|
Coding sequence change
|
c.1630C>T
|
c.1630C>T
|
c.328C>T
|
NA
|
c.553T>G
|
c.160C>T
|
c.160C>T
|
c.160C>T
|
c.328C>T
|
c.328C>T
|
c.328C>T
|
|
Amino acid change
|
R544C
|
R544C
|
R110C
|
NA
|
C185G
|
R54C
|
R54C
|
R54C
|
R110C
|
R110C
|
R110C
|
|
Exon
|
11
|
11
|
3
|
NA
|
4
|
2
|
2
|
2
|
3
|
3
|
3
|
|
Skin biopsy#
|
NA
|
NA
|
NA
|
NA
|
NA
|
GOM+
|
NA
|
NA
|
GOM+
|
GOM+
|
NA
|
|
* A - Genetic/ biopsy; B - Clinical +
MRI changes; C - Clinical (stroke/ TIA/ dementia/ psychiatric features); #
GOM - granular osmophilic material; NA - Not available
|
Table
3. Scheltens scores of the CADASIL
cases
|
Case
|
1
|
2
|
3
|
3.1
|
4
|
5
|
5.1
|
5.2
|
|
Periventricular hyperintensities 1 (PVH 0-6)
|
|
Capsular occipital
|
2
|
1
|
2
|
2
|
2
|
2
|
2
|
1
|
|
Capsular frontal
|
2
|
2
|
1
|
2
|
1
|
2
|
2
|
1
|
|
Bands lateral ventricle
|
2
|
1
|
1
|
1
|
2
|
2
|
2
|
1
|
|
White matter hyperintensities 2 (WMH 0-24)
|
|
Frontal
|
6
|
6
|
6
|
6
|
6
|
6
|
6
|
4
|
|
Parietal
|
6
|
6
|
1
|
6
|
6
|
6
|
6
|
4
|
|
Temporal
|
0
|
0
|
3
|
3
|
3
|
6
|
6
|
0
|
|
Occipital
|
6
|
0
|
1
|
5
|
6
|
0
|
6
|
0
|
|
Basal ganglia hyperintensities 2 (BG 0-30)
|
|
Caudate
|
3
|
1
|
0
|
0
|
1
|
3
|
0
|
0
|
|
Putamen
|
1
|
2
|
3
|
0
|
1
|
3
|
0
|
0
|
|
Globus pallidus (GP)
|
0
|
1
|
0
|
3
|
2
|
0
|
0
|
0
|
|
Thalamus
|
0
|
2
|
0
|
1
|
1
|
0
|
4
|
0
|
|
Internal capsule
|
1
|
1
|
3
|
2
|
3
|
3
|
3
|
3
|
|
Infratentorial foci of hyperintensity 2
(ITF 0-24)
|
|
Cerebellum
|
0
|
0
|
0
|
0
|
0
|
0
|
3
|
0
|
|
Midbrain
|
0
|
0
|
0
|
0
|
0
|
0
|
3
|
0
|
|
Pons
|
1
|
1
|
0
|
0
|
0
|
0
|
3
|
4
|
|
Medulla
|
0
|
1
|
0
|
0
|
0
|
0
|
3
|
0
|
|
Total
|
30
|
25
|
21
|
31
|
34
|
33
|
50
|
17
|
|
Other findings 3
|
|
Projection fibers (Internal capsule
posterior limb)
|
0
|
1
|
1
|
1
|
2
|
1
|
2
|
0
|
|
Temporal white matter
|
|
Anterior to posterior margin of amygdala
|
1
|
0
|
2
|
1
|
1
|
3
|
3
|
0
|
|
Posterior to posterior margin of amygdala
|
0
|
1
|
0
|
1
|
0
|
1
|
1
|
0
|
|
External capsule
|
1
|
2
|
1
|
2
|
3
|
3
|
3
|
0
|
|
Atrophy 4
|
1
|
1
|
0
|
0
|
1
|
1
|
2
|
0
|
1 0 = absent; 1 = 0 to 5 mm; 2 = > 5 mm
2 0 = absent; 1 = up to five lesions of <3mm
diameter; 2 = six or more lesions of <3mm; 3 = up to five lesions 4 to 10 mm
in diameter; 4 = six or more lesions of 4 to 10mm; 5 = one or more lesions 10mm
in size; and 6 = confluent hyperintensity
3 0 = absent, 1 = <5 lesions, 2 = 5-10 lesions, 3 =
>10 lesions
4 0 = absent, 1 = mild, 2 = moderate, 3 = severe
(sulcal, cerebellar folia prominence, enlargement of ventricles, brainstem
size)
3.1
Neuroimaging findings
Figure
2 shows the axial MRI features for cases 1, 3 and 4 with the involvement of
basal ganglia, anterior temporal pole and periventricular regions,
respectively. Scheltens scoring of the MRI brain for all cases are shown in
Table 3. The scoring for some family members and the cases reported by Wilder-Smith
et al. [5] were not included as there were either no MRI brain
or no access to the MRI images. All the available cases demonstrated T2-lesions
in the periventricular and white matter regions of the frontal and parietal
lobes, with the involvement of the internal capsule. All cases showed external
capsule involvement except an asymptomatic case (5.2). Cases 1, 2, 5.1 and 5.2
showed white matter changes of the infratentorial structures, namely brainstem
and cerebellum. Cases 1, 2 and 5.2 showed no involvement of the temporal pole.
All the subjects demonstrated normal MR angiographic findings with no evidence
of intracranial vascular stenosis seen. Cerebral atrophy was more prominent in
the subjects with the higher Scheltens score. Case 1, 3 and 4 showed no
blooming artefacts on gradient echo (GRE) or susceptibility weighted imaging
(SWI) sequences. These sequences were not available in other cases.

Figure 1. Pedigrees for the families affected by
CADASIL (cases 3-6). Square and circle denote male and female, respectively.
Solid square and circle denote affected male and female, respectively. Vertical
band denotes asymptomatic mutation carrier. The proband for each family is
denoted with an arrow. A strikethrough denotes a deceased member.
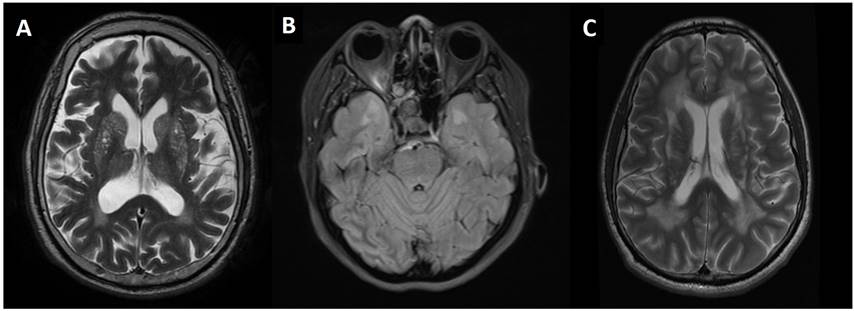
Figure 2. (A)
Axial T2W MRI brain of case 1 demonstrates hyperintensities in caudate nucleus,
lentiform nucleus. (B) Axial FLAIR sequence of case 3 demonstrates
anterior temporal pole involvement. (C) Axial T2W MRI brain of case 4
demonstrates confluent deep white matter and periventricular lesions.
3.2
NOTCH3 mutations
We
sequenced exons 2 to 24 of NOTCH3 of the proband. Exons 2-24 encode 34 EGFr
domains of NOTCH3 [11]. Three different cysteine-altering missense mutations
were identified from Case 1 - 4 (Figure 3). In our series, two Chinese patients
had a heterozygous mutation in exon 11 with c.1630C>T (p.Arg544Cys, R544C).
One family with mixed Indian and Chinese ancestry had a heterozygous mutation
in exon 3 with c.328C>T (p.Arg110Cys, R110C). Another Indian family was
detected to have a heterozygous mutation in exon 4 with c.553T>G
(p.Cys185Gly, C185G).
4.0 DISCUSSION
This
series included four different mutations in exon 2, 3, 4 and 11 of the NOTCH3
gene from 3 different ethnic groups with diverse phenotypic variations
including the age of onset and presenting symptoms. Some studies reported a
weak genotype-phenotype correlation for CADASIL, even within the same pedigree [12,13]. Other modulating factors, such as hypertension and smoking have been
shown to influence disease severity [12]. Despite the diverse phenotypic variations, a few
mutations have been associated with certain unique phenotypes. Opherk et al.
[14] demonstrated that the C117F mutation was associated
with earlier mortality and C174Y mutation with a lower age of stroke onset. The
R153C mutation was significantly
associated with the presence of microbleeds [15]. A worse profile of white matter lesions has been
noted in the C440G mutation [16]. Another mutation that has been associated with its
own unique phenotype is R544C. Other than the older age of onset, R544C
mutation is associated with a higher incidence of intracranial haemorrhage,
rarer occurrence of migraine, a higher percentage of cognitive dysfunction and
lower frequency of anterior temporal pole involvement [3,4,7]. These features
can also be seen in our cases with R544C mutation where both cases showed later
age of onset and no migraine. The MRI for these two patients also showed a lack
of anterior temporal pole involvement.
The
R544C mutation is highly prevalent in the CADASIL cohorts reported in Taiwan
(70.5%) [4] and Jeju Island of Korea (75-90.3%) [3,17]. It also accounts for a small
proportion of CADASIL patients in mainland Korea (27.6%) [18] and China (2.3-15.5%) [2,6,19]. Chen et al.
[2] reported that the patients carrying R544C mutation in
Taiwan and Fujian (China) might be descendants from a common ancestor. The high
incidence of R544C in the island of Jeju is also postulated to be due to the
founder effect.

Figure 3. Mutation
analysis of the NOTCH3 gene by
direct DNA sequencing. Partial sequencing chromatograms (forward and reverse
strand) revealed the heterozygous missense mutation in Case 1-4.
The
family tree for Case 3, who is of mixed Chinese and Indian ancestry shows that
the origin of the mutation is likely from the maternal Chinese side of the
family. Out of the four Chinese families in this series, a high proportion
(50%) of them had R544C mutation. There seemed to be a suggestion of a link
between the Malaysian Chinese and the population of Han Chinese in Taiwan,
Fujian and the Koreans in Jeju Island, though this remains a postulate as the
number of patients in this series is low. Interestingly, both our R544C
patients are of Hakka dialect while the majority of Han Chinese in Taiwan/
Fujian are of Hokkien dialect. Cross marriage between various Chinese dialect
groups is common in Southeast Asian countries, including Malaysia, which may
explain the finding.
There
are also two families with South Asian ancestry included in our series, with
mutations in R110C (exon 3, case 3) and C185G (exon 4, case 4), of which both
mutations had been previously reported in European populations [14,20]. To the best of our knowledge, 22 cases (a family of 17 members and five
other individual cases) of CADASIL have been reported in individuals with South
Asian ancestry (Indian and Sri Lankan) so far. Out of those, only eight were
genetically confirmed. The mutations found included R141C mutation (exon 4) in
six cases from two families, C260G (exon 5) and C144S (exon 4) in one case each
[21-26]. The relatively lower number of genetically confirmed cases reported
from the South Asian region could be due to under-recognition of the condition
and the difficulty in access to genetic screening.
This
case series has several limitations. The number of cases is small due to single
centre recruitment and the lack of prospective screening protocol, thus
limiting a broader application of the results from this study.
The family pedigree is incomplete for case 1 and 3, due to patients'
difficulties in providing detailed information on their family history. Due to the
retrospective nature of the study, specific MRI sequences were not obtained and
resulted in an incomplete analysis.
5.0 CONCLUSION
This
case series highlights the various genotype and phenotype spectrum in our
multiethnic country. A more extensive study, including more positive cases, is
needed to further map out the different CADASIL genotypes in Malaysia.
Supplementary Materials: The questionnaire
used for obtaining clinical data from the proband and family members is available online at https://doi.org/10.31117/neuroscirn.v2i3.35.
Acknowledgements: This work was supported
by University of Malaya Research Grant (RG517-13HTM), University of Malaya, to K.-S.L.
and High Impact Research Grant (UM-MOHE UM.C/625/1/HIR/MOHE/ CHAN-02 H-50001-A000023),
University of Malaya, to C.-C.N. We thank the affected individuals and their
families for participating in this study.
Author Contributions: T.-H.T., K.-S.L. and C.-C.N. contributed in the acquisition,
interpretation of data for the work, drafting, critical revision and final
approval. I.I., S.B.A., T.-T.L., I.L., A.-H.T., C.-K.C., C.-S.L. had
substantial contributions to the acquisition, interpretation of data for the
work and critical revision. C.-T.T. had substantial contributions to the
conception or design of the work, analysis, or interpretation of data for the
work, critical revision, and final approval.
Conflicts of Interest: Funding
sponsors had no role in the study design, collection, analysis and
interpretation of data, writing of the report and in the decision to submit the
article for publication.
References
1.
Stojanov D, Vojinovic S, Aracki-Trenkic A, Tasic A,
Benedeto-Stojanov D, Ljubisavljevic S, et al. Imaging characteristics of
cerebral autosomal dominant arteriopathy with subcortical infarcts and
leucoencephalopathy (CADASIL). Bosn J Basic Med Sci. 2015;15(1):1-8. https://doi.org/10.17305/bjbms.2015.247
2.
Chen S, Ni W, Yin X-Z, Liu H-Q, Lu
C, Zheng Q-J, et al. Clinical features and mutation spectrum in Chinese
patients with CADASIL: A multicenter retrospective study. CNS Neurosci Ther.
2017;23(9):707-716. https://doi.org/10.1111/cns.12719
3.
Choi JC, Kang S-Y, Kang J-H, Park
J-K. Intracerebral hemorrhages in CADASIL. Neurology. 2006;67(11):2042-2044.
https://doi.org/10.1212/01.wnl.0000246601.70918.06
4.
Liao Y-C, Hsiao C-T, Fuh J-L,
Chern C-M, Lee W-J, Guo Y-C, et al. Characterization of CADASIL among the Han
Chinese in Taiwan: Distinct Genotypic and Phenotypic Profiles. PLoS ONE.
2015;10(8):e0136501. https://doi.org/10.1371/journal.pone.0136501
5.
Wilder-Smith E, Shen Y, Ng YK, Yu
GX, Chew NK, Tan CT, et al. Cerebral autosomal dominant arteriopathy with
subcortical infarcts and leukoencephalopathy (CADASIL) in a Chinese family:
clinical, radiological and skin biopsy features. J Clin Neurosci.
2004;11(3):304-307. https://doi.org/10.1016/j.jocn.2003.05.007
6.
Liu X, Zuo Y, Sun W, Zhang W, Lv
H, Huang Y, et al. The genetic spectrum and the evaluation of CADASIL screening
scale in Chinese patients with NOTCH3 mutations. J Neurol Sci.
2015;354(1-2):63-69. https://doi.org/10.1016/j.jns.2015.04.047
7.
Lee Y-C, Liu C-S, Chang M-H, Lin K-P,
Fuh J-L, Lu Y-C, et al. Population-specific spectrum of NOTCH3 mutations, MRI
features and founder effect of CADASIL in Chinese. J Neurol.
2009;256(2):249-255. https://doi.org/10.1007/s00415-009-0091-3
8.
Lim K-S, Tan A-H, Lim C-S, Chua K-H,
Lee P-C, Ramli N, et al. R54C Mutation of NOTCH3 Gene in the First Rungus
Family with CADASIL. PLoS ONE. 2015;10(8):e0135470. https://doi.org/10.1371/journal.pone.0135470
9.
Scheltens P, Barkhof F, Leys D,
Pruvo JP, Nauta JJ, Vermersch P, et al. A semiquantative rating scale for the
assessment of signal hyperintensities on magnetic resonance imaging. J
Neurol Sci. 1993;114(1):7-12. https://doi.org/10.1016/0022-510x(93)90041-v
10.
Rutten JW, Haan J, Terwindt GM,
van Duinen SG, Boon EMJ, Oberstein SAJL. Interpretation of NOTCH3 mutations in
the diagnosis of CADASIL. Expert Rev Mol Diagn. 2014;14(5):593-603. https://doi.org/10.1586/14737159.2014.922880
11.
Rutten JW, Dauwerse HG,
Gravesteijn G, van Belzen MJ, van der Grond J, Polke JM, et al. Archetypal
NOTCH3 mutations frequent in public exome: implications for CADASIL. Ann
Clin Transl Neurol. 2016;3(11):844-853. https://doi.org/10.1002/acn3.344
12.
Adib-Samii P, Brice G, Martin RJ,
Markus HS. Clinical spectrum of CADASIL and the effect of cardiovascular risk
factors on phenotype: study in 200 consecutively recruited individuals. Stroke.
2010;41(4):630-634. https://doi.org/10.1161/STROKEAHA.109.568402
13.
Dichgans M. Cerebral autosomal
dominant arteriopathy with subcortical infarcts and leukoencephalopathy:
phenotypic and mutational spectrum. J Neurol Sci. 2002;203-204(Special_Issue):77-80.
https://doi.org/10.1016/s0022-510x(02)00270-8
14.
Opherk C, Peters N, Herzog J,
Luedtke R, Dichgans M. Long-term prognosis and causes of death in CADASIL: a
retrospective study in 411 patients. Brain. 2004;127(Pt 11):2533-2539. https://doi.org/10.1093/brain/awh282
15.
Oberstein SAL, van den Boom R, van
Buchem MA, van Houwelingen HC, Bakker E, Vollebregt E, et al. Cerebral
microbleeds in CADASIL. Phys Ther. 2001;57(6):1066-1070. https://doi.org/10.1212/wnl.57.6.1066
16.
Singhal S, Bevan S, Barrick T,
Rich P, Markus HS. The influence of genetic and cardiovascular risk factors on
the CADASIL phenotype. Brain. 2004;127(Pt 9):2031-2038. https://doi.org/10.1093/brain/awh223
17.
Choi JC, Song S-K, Lee JS, Kang S-Y,
Kang J-H. Diversity of stroke presentation in CADASIL: study from patients
harboring the predominant NOTCH3 mutation R544C. J Stroke Cerebrovasc Dis.
2011;22(2):126-131. https://doi.org/10.1016/j.jstrokecerebrovasdis.2011.07.002
18.
Kim Y-E, Yoon CW, Seo SW, Ki C-S,
Kim YB, Kim J-W, et al. Spectrum of NOTCH3 mutations in Korean patients with
clinically suspicious cerebral autosomal dominant arteriopathy with subcortical
infarcts and leukoencephalopathy. J Neurol Sci. 2013;35(3):726.e1-6. https://doi.org/10.1016/j.neurobiolaging.2013.09.004
19.
Tan Q-C, Zhang J-T, Cui R-T, Xu Q-G,
Huang X-S, Yu S-Y. Characteristics of CADASIL in Chinese mainland patients. Neurol
India. 2014;62(3):257-261. https://doi.org/10.4103/0028-3886.136900
20.
Joutel A, Favrole P, Labauge P,
Chabriat H, Lescoat C, Andreux F, et al. Skin biopsy immunostaining with a
Notch3 monoclonal antibody for CADASIL diagnosis. Lancet.
2002;358(9298):2049-2051. https://doi.org/10.1016/S0140-6736(01)07142-2
21.
Panagariya A, Sharma B,
Shubhakaran. CADASIL in a family from north-west India. J Assoc Physicians
India. 2004;52:580-581. https://www.ncbi.nlm.nih.gov/pubmed/15645988
22.
Gurumukhani JK, Ursekar M, Singhal
BS. Cerebral autosomal dominant arteriopathy with subcortical infarcts and
leucoencephalopathy (CADASIL): a case report with review of literature. Neurol
India. 2004;52(1):99-101. https://www.ncbi.nlm.nih.gov/pubmed/15069251
23.
Yadav S, Bentley P, Srivastava P,
Prasad K, Sharma P. The first Indian-origin family with genetically proven
cerebral autosomal dominant arteriopathy with subcortical infarcts and
leukoencephalopathy (CADASIL). J Neurol Sci. 2011;22(1):28-31. https://doi.org/10.1016/j.jstrokecerebrovasdis.2011.05.023
24.
Nandhagopal R. CADASIL - Cerebral
Autosomal Dominant Arteriopathy with Subcortical Infarcts and
Leukoencephalopathy. Sultan Qaboos Univ Med J. 2011;11(2):284-285.
25.
De Silva KRD, Gamage R, Dunuwille
J, Gunarathna D, Sirisena D, Weerasinghe A, et al. Cerebral autosomal dominant
arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL): a
patient from Sri Lanka. J Neurol Sci. 2009;16(11):1492-1493. https://doi.org/10.1016/j.jocn.2009.01.019
26.
Eswaradass VP, Ramasamy B,
Kalidoss R, Gnanagurusamy G. Cadasil coma: Unusual cause for acute
encephalopathy. Ann Indian Acad Neurol. 2015;18(4):483-484. https://doi.org/10.4103/0972-2327.160072
![]()


