
Zinc transporter-3
[SLC30A3 (rs11126936)] polymorphism is associated with major depressive disorder
in Asian subjects
Munn-Sann Lye 1*,
Aishah-Farhana Shahbudin 1, Yin-Yee Tey 1, Yin-Sim Tor 1,2,
King-Hwa Ling 3, Normala Ibrahim 4, Johnson Stanslas 5,
Su-Peng Loh 6 and Rozita Rosli 3
1 Department of Community Health, Faculty of
Medicine and Health Sciences, Universiti Putra Malaysia, 43400 UPM Serdang,
Selangor, Malaysia.
2 School of Bioscience, Faculty of Health and
Medical Sciences, Taylor's University, 47500 Subang Jaya, Selangor, Malaysia.
3 Department of Biomedical Science, Faculty
of Medicine and Health Sciences, Universiti Putra Malaysia, 43400 UPM Serdang,
Selangor, Malaysia.
4 Department of Psychiatry, Faculty of
Medicine and Health Sciences, Universiti Putra Malaysia, 43400 UPM Serdang,
Selangor, Malaysia.
5 Pharmacotherapeutics Unit, Department of
Medicine, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, 43400
UPM Serdang, Selangor, Malaysia.
6 Department of Nutrition and Dietetics,
Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, 43400 UPM Serdang,
Selangor, Malaysia.
* Correspondence: lyems9@yahoo.com; Tel.: +60-12-308-3186
Received: 10 May 2019; Accepted: 19 August 2019; Published: 13
September 2019
Edited by: Norshariza Nordin (Universiti Putra Malaysia,
Malaysia)
Reviewed by: Rushita Bagchi (University of Colorado, USA); Azlina
Ahmad Annuar (University of Malaya, Malaysia)
https://doi.org/10.31117/neuroscirn.v2i3.34
ABSTRACT: Major depressive disorder (MDD) compromises the
individual's capacity for self-care and productivity. Single nucleotide
polymorphisms (SNP) of a number of genes have been associated with MDD. The
zinc transporter-3 protein, encoded by the ZnT3 (SLC30A3) gene,
maintains zinc-glutamate homeostasis at the glutamatergic synapse, a disruption
of which increases risk of MDD. We hypothesise that variation in SLC30A3 (rs11126936)
SNP increases risk of MDD. We recruited 300 MDD cases and 300 controls, matched
in the ratio of 1:1 by age, gender and ethnicity. PCR-restriction fragment
length polymorphism analysis was used in DNA genotyping, validated by
sequencing 10% of samples. Deviation from the
Hardy-Weinberg equilibrium was tested using the chi-square test. Conditional
logistic regression was used to estimate adjusted odds ratios, controlling for
age, gender, ethnicity, occupation and family monthly income. Genotypes G/G and G/T showed two times greater
odds of developing MDD compared to variant genotype T/T (OR=1.983, 95% CI=1.031-3.815; p=0.040 and OR=2.232, 95%
CI=1.100-4.533; p=0.026 respectively). Carriers of genotypes G/G and G/T of the
SNP rs11126936 in SLC30A3 are associated with increased risk of MDD.
Keywords: depression;
genetics; mood disorders; biological markers; zinc transporter (ZnT3) gene; SLC30A3
(rs11126936) SNP;
©2019 by Lye et al. for use and distribution in accord with the
Creative Commons Attribution (CC BY-NC 4.0) license (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted
non-commercial use, distribution, and reproduction in any medium, provided the
original author and source are credited.
1.0 INTRODUCTION
Major depressive disorder (MDD) is a chronic disorder
associated with high rates of non-recovery, recurrence and comorbidity [1]. DSM-5 defines MDD
as "discrete episodes of at least 2 weeks' duration with clear-cut changes in
affect, cognition, and neuro-vegetative functions, and inter-episode
remissions" [2]. MDD
adversely affects psychosocial function associated with reduced work
productivity and days lost at work [2] and
is the leading cause of disability in developed and developing countries,
contributing to substantial health and economic burdens [3,4].
Depression when severe enough, compromises one's capability of self-care and
independent living [5] and
results in suicidal attempts in 4% of 19,723 inpatients reported in a
systematic review [6].
The prevalence of MDD varies between 11.1% to 23.0%
across different regions and populations [7-10].
According to the World Health Organization, 3.8% of the Malaysian population is
affected by depressive disorders [4].
Local studies have reported the prevalence of depressive illness to be between
10.3% to 32.7% [11-15].
Genetic influence on the development of MDD is
indicated by the heritability of between 40 to 75% [16-21].
Single nucleotide polymorphisms of a number of genes such as tryptophan
hydroxylase-1 (TPH1), tryptophan hydroxylase-2 (TPH2) gene and brain-derived
neurotrophic factor (BDNF) gene have been associated with MDD [22-26]. A
point mutation in the SLC30A3 gene, particularly rs11126936 SNP,
has been shown to be associated with schizophrenia in genome-wide association
studies [27] and
low blood zinc levels [28].
The SLC30A3 gene
encodes for the zinc transporter-3 (ZnT3) protein [29], which "is the sole mechanism
for concentrating zinc ions within synaptic vesicles in a subset of the brain's
glutamatergic neurons" [30]. Zinc efflux and influx, controlled by zinc transporter genes, play a
crucial role in zinc-glutamate homeostasis at the synaptic junction, which
modulates the balance of excito-inhibitory impulses of the glutamatergic
neurons [31,32]. Lower levels of ZnT3 have been found on autopsy in
suicidal cases diagnosed with MDD [33]. Although there is extensive
literature on blood zinc level and MDD, none have described the relationship
between genetic variation of ZnT3 and risk of MDD. This study aims to elucidate
the effect of ZnT3 (SLC30A3 rs11126936) SNP on risk of MDD.
2.0 MATERIALS AND METHODS
2.1
Ethics approval and consent to participate
This
study was approved by the Medical Research and Ethics Committee of the Ministry
of Health Malaysia (NMRR No.: NMRR-14-688-19696). All recruited subjects gave
written informed consent to participate.
2.2 Study design and subject recruitment
Three hundred case-control pairs matched on a 1:1
ratio by age (±5 years), gender and ethnicity, were recruited from psychiatry
clinics in four public hospitals from 2014 to 2017. Cases included those who
were 18 to 65 years of age diagnosed with single-episode non-psychotic Major
Depressive Disorder (using the Diagnostic and Statistical Manual of Mental
Disorders, Fifth Edition (DSM-5)) who presented with a history of MDD less than
2 years prior to recruitment.
Patients
who had (i) significant suicidal risk as assessed by the psychiatrist at the
point of inclusion, or diagnosed with (ii) dementia (iii) schizophrenia or
other psychotic disorder (iv) bipolar I or II disorder, and (v) anxiety
disorders including panic disorder, generalized anxiety disorder,
obsessive-compulsive disorder and post-traumatic stress disorder were excluded
from the study. Controls without a history of psychiatric disorders were
recruited from patients attending otorhinolaryngology and ophthalmology clinics
from the same hospitals where the cases were recruited. The sample size was estimated
based on a power of 80%, and a level of significance of 0.05 to detect an odds
ratio of 2 or greater [34].
2.3
Blood Collection
Using evacuated EDTA tubes (Vacutainer Tubes,
Becton-Dickinson, USA), 5 ml of venous blood were collected from each subject
and stored at 4°C prior to genomic DNA extraction within 24 h.
2.4 Genotyping of SLC30A3 rs11126936
SNP
Genomic
DNA was extracted from the buffy coat of the collected whole blood by using
QIAamp DNA Mini and Blood Mini kit in accordance with the manufacturer's
protocol (Qiagen, USA). Purity was determined using a NanoPhotometer® Classic
(Implen, USA) and the acceptable range for the ratio A260/A280 was 1.8-2.0.
Genotyping
protocol was adapted from Fujihara et al. 2018 [28]. Oligonucleotide primers (both
sense-F 5′-TCCCAGAACCTCCACTCCTGGATCCTG-3′ and antisense-R
3'-CCCCAGCTCTGGAATCTAGCCATCAGTTCT-5') were used to amplify the targeted SNP.
Genotyping of SLC30A3 rs11126936 was performed using polymerase chain
reaction-restriction fragment length polymorphism (PCR-RFLP). A region of 146
bp carrying the polymorphic restriction sites of MspI was amplified in a
20 μl PCR cocktail containing 100 ng of genomic DNA. PCR composition, PCR
condition, and RFLP digestion are listed in Table 1. Amplified fragments were
subjected to RFLP analysis. Using the restriction endonuclease MspI,
which cuts at 5'...C^CGG...3', the variant (T/T) genotype [35] produced a single undigested
product with 146 bp, G/T genotype produced partially digested product with
146 bp, 109 bp and 37bp, while G/G genotype was fully digested into
109 bp and 37 bp fragments.
Ten percent of randomly selected PCR products were
sequenced using ABI PRISM 3730xl Genetic Analyzer (Thermo Scientific, USA) to
confirm accuracy of the genotyping method. The cycle sequencing reaction was
performed following manufacturer's specifications (BigDye® Terminator v3.1
Cycle Sequencing Kit, Thermo Scientific, USA). The DNA sequence was then viewed
on a sequence analysis software (Sequence Scanner Software 2.0, Thermo
Scientific, USA).
Table
1. PCR composition, PCR condition,
RFLP digestion of SLC30A3 rs11126936 polymorphisms
|
Polymorphisms
|
SLC30A3 (rs11126936)
|
|
PCR
composition
|
20
μL of total PCR reaction consisted of 1X DreamTaq green PCR mastermix
with DreamTaq DNA polymerase, 1X DreamTaq Green buffer, dATP (0.4 mM), dCTP
(0.4 mM), dGTP (0.4 mM) and dTTP (0.4 mM), and 4 mM MgCl2 (Thermo
Fisher Scientific, USA), 10 μM of each primer 100 ng of genomic DNA and nuclease free water.
|
|
PCR
condition
|
94oC
for 3 minutes, followed by 30 cycles of denaturation at 96oC for 1
minute, annealing at the melting temperature 63oC for 1 minute and
extension at 72oC for 1 minute, with a final 10 min extension at
72oC and stored at 4oC.
|
|
RFLP
digestion
|
15
μL of total reaction mixture consisting of ~8 μL of PCR product, 1X
CutSmart® Buffer, 20 U of MspI (New England Biolabs, USA) was prepared
in nuclease-free water. The mixture was incubated at 37oC for 2
hours followed by 15 minutes at 65oC.
|
2.5
Data analysis
McNemar
test was used to determine differences in sociodemographic variables between
cases and controls using IBM Statistical Package for the Social Sciences (IBM
SPSS) version 22. Conditional logistic regression (using STATA 10) was used to
estimate odds ratios and 95% confidence intervals (CIs), controlling for
confounding variables of age, gender, ethnicity, occupation, education and
family monthly income. Statistical significance was set at alpha=0.05. Court
Lab Calculator [36] was used to perform the
chi-square test for deviations from Hardy-Weinberg equilibrium (HWE) for SLC30A3
(rs11126936) gene polymorphism in the controls.
3.0 RESULTS
3.1 Socio-demographic characteristics of cases and controls
Significant
differences were found between cases and controls (p<0.05) in educational
level, occupation, monthly income and family history of psychiatric illnesses.
A higher proportion of controls (70.3%) obtained tertiary education compared to
cases (51.0%) while MDD cases had lower monthly family incomes, where half of
them earned less than RM 2,000 (USD 497.00) per month. In contrast to controls
(4.7%), a much higher proportion (26.7%) of MDD cases had a family history of
psychiatric illnesses while a greater proportion of controls worked in
government or semi-government sectors (40.3%) (Table 2).
3.2
Laboratory findings
The
representative images of the original gels are shown in Figure 1A. Ten percent
of total samples from each genotype sent for sequencing were 100 % identical
with the results of PCR-RFLP. Figure 1B shows the partial sequence
chromatograms of SLC30A3 (rs11126936) polymorphism.
The
genotypic distribution of SLC30A3 rs11126936 polymorphism for controls
was T/T: 19% (57/300); G/T: 23% (69/300); G/G: 58% (174/300) while for cases,
it was T/T: 12.7% (38/300); G/T: 31.7% (95/300); G/G: 55.7% (167/300). SLC30A3
rs11126936 polymorphism deviated from Hardy-Weinberg Equilibrium (p
<0.001). Conditional logistic
regression showed that subjects with genotypes G/T (OR=2.23, 95% CI=1.10 - 4.53; p=0.026) and G/G (OR=1.98, 95% CI=1.03 - 3.82;
p=0.040) had two times higher odds of developing major depressive disorder
compared to subjects with variant genotype T/T after adjusting for age, gender,
ethnicity, occupation, education and family monthly income. Those without a
family history experienced a fifth of the odds of MDD (OR=0.18, 95% CI=0.09 -
0.36; p=<0.001) compared with those with a family history (Table 3).
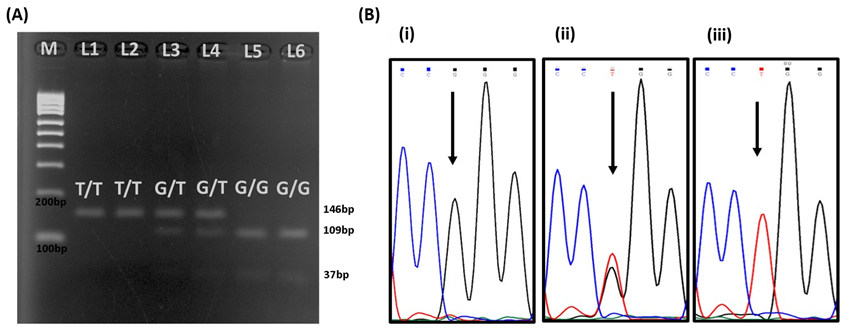
Figure 1. (A) Gel
electrophoresis of PCR-RFLP products for SLC30A3 rs11126936
polymorphisms. M on the figure represents DNA ladder marker; L1 and L2
represent T/T genotype (146bp), L3 and L4 represent G/T genotype (146 bp, 109 bp
and 37 bp); L5 and L6 represent G/G genotype (109 bp and 37 bp). (B) Partial
sequence chromatograms of SLC30A3 rs11126936 polymorphism from study
subjects. Arrow indicates the location of the nucleotide changes. Partial
sequence chromatogram (i) represents G/G genotype; (ii) represents G/T
genotype; (iii) represents T/T genotype.
Table 2. Sociodemographic characteristics
of the study population.
|
Variables
|
Cases, n=300%
|
Controls, n=300 (%)
|
χ 2
|
P value
|
|
Age (years)
18-25
26-35
36-45
46-55
56-65
|
52
76
83
48
41
|
(17.3)
(25.3)
(27.7)
(16.0)
(13.7)
|
55
89
62
55
39
|
(18.3)
(29.7)
(20.7)
(18.3)
(13.0)
|
4.675
|
0.320
|
|
Gender
Male
Female
|
97
203
|
(32.3)
(67.7)
|
97
203
|
(32.3)
(67.7)
|
0.000
|
1.000
|
|
Ethnicity
Malay
Chinese
Indian
and others
|
149
90
61
|
(49.7)
(30.0)
(20.3)
|
149
90
61
|
(49.7)
(30.0)
(20.3)
|
0.000
|
1.000
|
|
Family income
< RM 1000
RM1001-RM2000
RM2001-RM3000
RM3001-RM4000
>RM4000
|
87
65
64
25
59
|
(29.0)
(21.7)
(21.3)
(8.3)
(19.7)
|
28
57
74
51
90
|
(9.3)
(19.0)
(24.7)
(17.0)
(0.0)
|
46.863
|
<0.001a
|
|
Education
level
Primary/Secondary
Certificate
Diploma
Degree/Postgraduate
|
147
25
45
83
|
(49.0)
(8.3)
(15.0)
(27.7)
|
89
27
82
102
|
(29.7)
(9.0)
(27.3)
(34.0)
|
27.062
|
<0.001a
|
|
Family History
No
Yes
|
220
80
|
(73.3)
(26.7)
|
286
14
|
(95.3)
(4.7)
|
54.949
|
<0.001a
|
|
Occupation
Private
Government/
Semi-government
Student
Retired
Others
|
85
47
41
29
98
|
(28.3)
(15.6)
(13.7)
(9.7)
(32.7)
|
66
121
64
23
26
|
(22.0)
(40.3)
(21.3)
(7.7)
(8.7)
|
78.640
|
<0.001a
|
a p < 0.05.
Table 3. Association of single nucleotide polymorphism of ZnT3 gene (rs11126936),
with MDD using conditional logistic regression (239 cases and 263 controls).
|
Variables
|
|
Crude OR
|
95% CI
|
p
|
ORa
|
95% CI
|
p
|
|
SLC30A3
(rs11126936)
|
T/Tb T/G
G/G
|
1
2.1816
1.4075
|
(1.2152 - 3.9165)
(0.8345 - 2.3739)
|
0.009*
0.200
|
1
2.2324
1.9834
|
(1.0995 - 4.5326)
(1.0311 - 3.8150)
|
0.026c
0.040c
|
|
Family history
|
Yes
No
|
1
0.1430
|
(0.0754 - 0.2714)
|
<0.001*
|
1
0.1771
|
(0.0867 - 0.3620)
|
<0.001c
|
a
odds ratio, adjusted by age,
gender, ethnicity, family monthly income, occupation and education by
conditional logistic regression.
b Variant genotype (T/T) as reference for SLC30A3
genotypes.
c p<0.05.
4.0 DISCUSSION
SLC30A3
rs11126936 (ZnT3 protein) is found
abundantly in the brain, mostly in areas regulating emotions where
glutamatergic neurons are distributed; namely the hippocampus, amygdala and
frontal cortex [31,37-39]. It was found
that genotypes G/G and G/T was associated with twice the odds of MDD compared
to the variant T/T. Fujihara et al. (2018) [28] postulated that
increased expression of the SLC30A family of genes might increase blood
zinc levels. This is plausible as SLC30A3 rs11126936 causes efflux of
zinc ions out of the neurons, thus increasing zinc in the extracellular space. Fujihara
et al. also found that the G/G genotype was associated with reduced
blood zinc concentration [28].
A considerably higher proportion (26.7%) of MDD cases
had a positive family history of psychiatric illnesses compared to 4.7% in
controls (χ2=54.949, p<0.01). Sullivan et al.
concluded in their meta-analysis that MDD is a familial disorder with
heritability between 40% to 75% [16,17,19-21]. Although SLC30A3 (rs11126936)
SNP was not consistent with HWE, this is unlikely to be due to genotyping assay
error as 10% of samples sequenced were in 100% concordance with RFLP results
and the distribution for the genotypes of the SNP mentioned above in controls
does not deviate towards an excess of heterozygotes [40]. Secondly, we do not think this
is due to selection bias, as care was taken to recruit controls from the same
hospitals as the cases so that the characteristics of controls would reflect
those of the population from which cases arose. Thirdly, the deviation from HWE
is likely due to "non-random mating" effect - leading to genetic
segregation in which marriages within the same ethnicity or cultural background
are still widely being practised in Malaysia [41]. Thus, the deviation from HWE
does not preclude further analysis as the SNP may not be in equilibrium due to
unidentified association with some other traits [40].
One
of the potential limitations of the study could be selection bias introduced by
the case-control study design. We have attempted to minimize this bias by
recruiting controls from the same hospitals as the cases; differences in terms
of sociodemographic characteristics were minimized by recruiting cases and
controls matched by age, gender and ethnicity and also, by controlling for age,
gender, ethnicity, family income, occupation and education by regression at the
stage of data analysis.
5.0 CONCLUSIONS
Carriers
of genotypes G/G and G/T of the SLC30A3 gene conferred twice the odds of
MDD compared to the variant T/T. A
significantly higher proportion (26.7%) of MDD cases had a positive family
history of psychiatric illnesses compared to 4.7% in controls. This study is an important first step to uncovering
the effect of SLC30A3 (rs11126936) polymorphism on the risk of MDD. To
the best of our knowledge, this is the first report of SLC30A3 (rs11126936)
SNP increasing risk of MDD.
Acknowledgements: We
thank the Director General of Health Malaysia for approval to publish this
manuscript. We also thank Dr Azizul Awaluddin, Dr Sharifah Suziah
Syed Mokhtar, Dr Mazni Mat Junus, Dr Elinda Tunan, Dr Ibrahim Mohammed Badamasi, Ms Aldoghachi Asraa Faris
Abdulridha, Mr Khairul Aiman Bin Lokman, Ms Nurul Asyikin Abdul Razaq, Ms Siti
Zubaidah Redzuan, Dr Vaidehi Ulanganathan, research assistants as well as the staff of the hospitals involved. The study
was funded by Research Management Centre Universiti Putra Malaysia
(GP-IPB/2013/9415700).
Author Contributions: M.S.L., K.H.L, N.I., J.S., S.P.L. and R.R. conceived
and designed the experiments and obtained funding; A.S. and Y.S.T. performed the experiments; Y.Y.T and M.S.L analyzed
the data; M.S.L, K.H.L, N.I., J.S. and
S.P.L. contributed reagents/materials/analysis
tools; M.S.L, Y.Y.T, Y.S.T. and A.S. wrote the paper.
Conflicts of Interest: The authors declare no conflict of interest.
References
1. Greden JF. The burden of recurrent
depression: causes, consequences, and future prospects. J Clin Psychiatry.
2001;62 Suppl 22(suppl 22):5-9.
2. Diagnostic and Statistical Manual of Mental
Disorders (DSM-5®). Fifth edition. American Psychiatric Publishing,
Incorporated; 2013. pp. 991, ISBN: 978-0-89042-554-1.
3. Collins PY, Insel TR, Chockalingam A, Daar
A, Maddox YT. Grand challenges in global mental health: integration in
research, policy, and practice. PLoS Med. 2013;10(4):e1001434. https://doi.org/10.1371/journal.pmed.1001434
4. World Health Organization. Depression and
other common mental disorders: global health estimates. 2017.
5. Fried EI, Nesse RM. The impact of
individual depressive symptoms on impairment of psychosocial functioning. PLoS
ONE. 2014;9(2):e90311. https://doi.org/10.1371/journal.pone.0090311
6. Bostwick JM, Pankratz VS. Affective
disorders and suicide risk: a reexamination. Am J Psychiatry.
2000;157(12):1925-1932. https://doi.org/10.1176/appi.ajp.157.12.1925
7. Patten SB, Williams JVA, Lavorato DH, Wang
JL, McDonald K, Bulloch AGM. Descriptive epidemiology of major depressive
disorder in Canada in 2012. Can J Psychiatry. 2015;60(1):23-30. https://doi.org/10.1177/070674371506000106
8. Fernandez-Pujals AM, Adams MJ, Thomson P,
McKechanie AG, Blackwood DHR, Smith BH, et al. Epidemiology and Heritability of
Major Depressive Disorder, Stratified by Age of Onset, Sex, and Illness Course
in Generation Scotland: Scottish Family Health Study (GS:SFHS). PLoS ONE.
2015;10(11):e0142197. https://doi.org/10.1371/journal.pone.0142197
9. Sund AM, Larsson B, Wichstrøm L. Prevalence
and characteristics of depressive disorders in early adolescents in central
Norway. Child Adolesc Psychiatry Ment Health. 2011;5:28. https://doi.org/10.1186/1753-2000-5-28
10. Avenevoli S, Swendsen J, He J-P, Burstein
M, Merikangas KR. Major depression in the national comorbidity
survey-adolescent supplement: prevalence, correlates, and treatment. J Am
Acad Child Adolesc Psychiatry. 2015;54(1):37-44.e2. https://doi.org/10.1016/j.jaac.2014.10.010
11. Tan KL, Yadav H. Depression among the urban
poor in Peninsular Malaysia: a community based cross-sectional study. J
Health Psychol. 2012;18(1):121-127. https://doi.org/10.1177/1359105311433908
12. Yee WS, Lin LP. Anxiety and depressive
symptoms among communities in the east coast of Peninsular Malaysia: A rural
exploration. Malays J Psychiatry. 2011;20(1).
13. Ibrahim N, Sherina MS, Phang CK, Mukhtar F,
Awang H, Ang JK, et al. Prevalence and predictors of depression and suicidal
ideation among adolescents attending government secondary schools in Malaysia. Med
J Malaysia. 2017;72(4):221-227.
14. Sidik SM, Arroll B, Goodyear F. Prevalence
of depression among women attending a primary urban care clinic in Malaysia. Singapore
Med J. 2012;53:468-473.
15. Maideen SFK, Sidik SM, Rampal L, Mukhtar F.
Prevalence, associated factors and predictors of depression among adults in the
community of Selangor, Malaysia. PLoS ONE. 2014;9(4):e95395. https://doi.org/10.1371/journal.pone.0095395
16. Bierut LJ, Heath AC, Bucholz KK, Dinwiddie
SH, Madden PA, Statham DJ, et al. Major depressive disorder in a
community-based twin sample: are there different genetic and environmental
contributions for men and women? Arch Gen Psychiatry. 1999;56(6):557-563.
https://doi.org/10.1001/archpsyc.56.6.557
17. Sullivan PF, Neale MC, Kendler KS. Genetic
epidemiology of major depression: review and meta-analysis. Am J Psychiatry.
2000;157(10):1552-1562. https://doi.org/10.1176/appi.ajp.157.10.1552
18. Levinson DF, Nichols WE. Major Depression
and Genetics - Genetics of Brain Function - Stanford University School of
Medicine. Available online: http://med.stanford.edu/depressiongenetics/mddandgenes.html (accessed on 26 June 2018)
19. Kendler KS, Pedersen NL, Neale MC, Mathé
AA. A pilot Swedish twin study of affective illness including hospital- and
population-ascertained subsamples: results of model fitting. Behav Genet.
1995;25(3):217-232.
20. McGuffin P, Katz R, Watkins S, Rutherford
J. A hospital-based twin register of the heritability of DSM-IV unipolar
depression. Arch Gen Psychiatry. 1996;53(2):129-136. https://doi.org/10.1001/archpsyc.1996.01830020047006
21. Kendler KS, Prescott CA. A population-based
twin study of lifetime major depression in men and women. Arch Gen
Psychiatry. 1999;56(1):39-44. https://doi.org/10.1001/archpsyc.56.1.39
22. Hao R, Qi Y, Hou D-N, Ji Y-Y, Zheng C-Y, Li
C-Y, et al. BDNF val66met Polymorphism Impairs Hippocampal Long-Term Depression
by Down-Regulation of 5-HT3 Receptors. Front Cell Neurosci.
2017;11(1):306. https://doi.org/10.3389/fncel.2017.00306
23. Lin Y-MJ, Ko H-C, Chang F-M, Yeh T-L, Sun
HS. Population-specific functional variant of the TPH2 gene 2755C>A
polymorphism contributes risk association to major depression and anxiety in
Chinese peripartum women. Arch Womens Ment Health. 2009;12(6):401-408. https://doi.org/10.1007/s00737-009-0088-z
24. Aldoghachi AF, Tor YS, Redzun SZ, Bin
Lokman KA, Razaq NAA, Shahbudin AF, et al. Screening of brain-derived
neurotrophic factor (BDNF) single nucleotide polymorphisms and plasma BDNF
levels among Malaysian major depressive disorder patients. PLoS ONE.
2019;14(1):e0211241. https://doi.org/10.1371/journal.pone.0211241
25. Wigner P, Czarny P, Synowiec E, Bijak M,
Białek K, Talarowska M, et al. Association between single nucleotide
polymorphisms of TPH1 and TPH2 genes, and depressive disorders. J Cell Mol
Med. 2018;22(3):1778-1791. https://doi.org/10.1111/jcmm.13459
26. Froud A, Murphy J, Cribb L, Ng CH, Sarris
J. The relationship between dietary quality, serum brain-derived neurotrophic
factor (BDNF) level, and the Val66met polymorphism in predicting depression. Nutr
Neurosci. 2017;22(7):513-521. https://doi.org/10.1080/1028415X.2017.1415281
27. Perez-Becerril C, Morris AG, Mortimer A,
McKenna PJ, de Belleroche J. Allelic variants in the zinc transporter-3 gene,
SLC30A3, a candidate gene identified from gene expression studies, show
gender-specific association with schizophrenia. Psychiatry.
2014;29(3):172-178. https://doi.org/10.1016/j.eurpsy.2013.05.007
28. Fujihara J, Yasuda T, Kimura-Kataoka K, Takinami
Y, Nagao M, Takeshita H. Association of SNPs in genes encoding zinc
transporters on blood zinc levels in humans. Leg Med (Tokyo).
2018;30(0):28-33. https://doi.org/10.1016/j.legalmed.2017.10.009
29.
Hediger MA,
Clémençon B, Burrier RE, Bruford EA. The ABCs of membrane transporters in
health and disease (SLC series): introduction. Mol Aspects Med.
2013;34(2-3):95-107. https://doi.org/10.1016/j.mam.2012.12.009
30. McAllister BB, Dyck RH. Zinc transporter 3
(ZnT3) and vesicular zinc in central nervous system function. Neurosci Biobehav Rev. 2017;80:329-350. https://doi.org/10.1016/j.neubiorev.2017.06.006
31. Petrilli MA, Kranz TM, Kleinhaus K, Joe P,
Getz M, Johnson P, et al. The Emerging Role for Zinc in Depression and
Psychosis. Front Pharmacol. 2017;8:414. https://doi.org/10.3389/fphar.2017.00414
32. Frederickson CJ, Suh SW, Silva D,
Frederickson CJ, Thompson RB. Importance of zinc in the central nervous system:
the zinc-containing neuron. J Nutr. 2000;130(5S Suppl):1471S-1483S. https://doi.org/10.1093/jn/130.5.1471S
33. McLoughlin IJ, Hodge JS. Zinc in depressive
disorder. Acta Psychiatr Scand. 1990;82(6):451-453. https://doi.org/10.1111/j.1600-0447.1990.tb03077.x
34. Xiao Z, Liu W, Gao K, Wan Q, Yang C, Wang
H, et al. Interaction between CRHR1 and BDNF genes increases the risk of
recurrent major depressive disorder in Chinese population. PLoS ONE.
2011;6(12):e28733. https://doi.org/10.1371/journal.pone.0028733
35. National Center for Biotechnology
Information. Reference SNP (refSNP) Cluster Report: rs11126936. Availabel
online: https://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=rs11126936. (accessed on 12 July 2018)
36. Court M. Court Lab Calculator. Available
online: https://www.researchgate.net/file.PostFileLoader.html?id=58dbf27a96b7e4fc194da329&assetKey=AS%3A477303651213312%401490809466020. (accessed
on 20 April 2018)
37. Paoletti P, Vergnano AM, Barbour B, Casado
M. Zinc at glutamatergic synapses. Neuroscience. 2008;158(1):126-136. https://doi.org/10.1016/j.neuroscience.2008.01.061
38. Mlyniec K. Zinc in the Glutamatergic Theory
of Depression. Curr Neuropharmacol. 2015;13(4):505-513.
39. Frederickson CJ. Neurobiology of zinc and
zinc-containing neurons. Int Rev Neurobiol. 1989;31:145-238.
40. Turner S, Armstrong LL, Bradford Y, Carlson
CS, Crawford DC, Crenshaw AT, et al. Quality control procedures for genome-wide
association studies. Curr Protoc Hum Genet. 2011;Chapter 1:Unit1.19. https://doi.org/10.1002/0471142905.hg0119s68
41. Nagaraj S. Intermarriage in Malaysia. Malays
J Econ Stud. 2009;46(1):75-92.
![]()
