
Chemical hypoxia in
human pluripotent NT2 stem cell-derived neurons: Effect of hydroxamic acid and
benzamide-based epigenetic drugs
Rushita A.
Bagchi 1,5,*, Ashim K. Bagchi 2,5, Ankita Salunke 3,
Dipak K. Hens 4 and Pragna H. Parikh 3
1 Department
of Medicine, University of Colorado Anschutz Medical Campus, Aurora, Colorado,
USA.
2
Institute of Cardiovascular Sciences, St. Boniface Albrechtsen Research Centre,
Winnipeg, Manitoba, Canada.
3 Department
of Zoology, The Maharaja Sayajirao University of Baroda, Vadodara, Gujarat,
India.
4 Sonamukhi
College, The University of Burdwan, West Bengal, India.
5 These
authors contributed equally to this work.
*
Correspondence: rush6782@gmail.com;
Tel.: +1-720-209-8586
Received: 31 March 2019; Accepted: 13 August 2019; Published: 22
August 2019
Edited by: King-Hwa Ling (Harvard Medical School, Boston, USA)
Reviewed by: Pike-See Cheah (Universiti Putra Malaysia, Malaysia);
Katharina Meyer (Harvard Medical School, Boston, USA); Kai-Leng Tan (Guangdong
University of Technology, China)
https://doi.org/10.31117/neuroscirn.v2i3.30
ABSTRACT: Hypoxia-induced oxidative
stress contributes to neuronal damage leading to many neurodegenerative
disorders. Hypoxia promotes many downstream effectors such as hypoxia-inducible
factor-1α (HIF-1α) in order to restore respiratory homeostasis due to
low oxygen availability and increased ROS. Use of histone deacetylase (HDAC)
inhibitors may modulate hypoxia-induced neuronal cell damage. In this study,
we used two chemically diverse HDAC inhibitors to investigate their effect on
hypoxia-exposed neuronal cells. Human pluripotent NT-2 stem cell-derived neuronal
differentiated cells were exposed to CoCl2 pre-treatment for 6h to
induce hypoxia, prior to supplementation of HDAC inhibitor (SAHA or MGCD0103).
Treatment with HDAC inhibitor improved cell viability in hypoxia-induced
neuronal cells. The increased HIF1α expression in hypoxia-induced neuronal
cells was blunted by these HDAC inhibitors with a concomitant decrease in ROS
production. CoCl2 treatment caused an increase in IL-1β, which
was significantly inhibited by these HDAC inhibitors. Furthermore, apoptosis
induced in these CoCl2 treated neuronal cells was mitigated by SAHA
as well MGCD0103 suggesting that these HDAC inhibitors are capable of reducing
cellular toxicity, inflammation and apoptosis, and thus, could be beneficial as
therapeutic molecules for many neuropathological conditions.
Keywords: pluripotent; neuron; hypoxia; histone deacetylase;
gene expression
©2019 by Bagchi et al. for use and distribution in accord with the
Creative Commons Attribution (CC BY-NC 4.0) license (https://creativecommons.org/licenses/by-nc/4.0/),
which permits unrestricted non-commercial use, distribution, and reproduction
in any medium, provided the original author and source are credited.
1.0 INTRODUCTION
Oxygen is an oxidizing agent; its homeostasis is
essential for maintaining physiological balance during cell development and
growth [1]. Under many pathological conditions, lack
of oxygen supply (known as hypoxia condition), interferes with energy
metabolism; this eventually causes cell death [2]. Neurological disorders occur due to
hypoxia-induced reactive oxygen species (ROS) production in neuronal cells [3]. The prolonged hypoxic condition may lead
to neuronal DNA damage, cell death and apoptosis, resulting in brain injury [4,5]. In hypoxic conditions, mitochondria release
apoptotic proteins such as cytochrome c (Cyt c) responsible for oxidative
phosphorylation and reduced ATP synthesis [6]. Increased Cyt c is associated with
increased ROS production due to decrease in antioxidant enzymes activity [7], which may lead to oxidative stress (OS).
Hypoxia is regulated by the hypoxia-inducible factor (HIF), a heterodimeric
transcription factor [8] that regulates oxygen homeostasis via the presence
of an oxygen-regulated subunit, HIF-1α [9]. Heme oxygenase 1 (HMOX1) plays an
important role in the antioxidant defense system and iron homeostasis [10]. In general, ROS production in the
mitochondria is responsible for stabilizing HIF-1α [11] and regulates HMOX1 [12]. Mitigation of OS can be achieved by
altering transcriptional regulation via epigenetic modifiers in order to cause
biased induction of cell survival pathways [13,14].
Pharmacological inhibition of these epigenetic
modifiers, histone deacetylases (HDACs) is vital for protection against many
pathological disorders. Alteration in these epigenetic factors may be a
beneficial approach to promote oxidative defense mechanism [15]. Effectiveness of HDAC inhibitors (HDACi)
has been shown to control many biological responses, including oxidative stress
and inflammatory responses, which in turn, affect downstream signaling and
systemic cellular functions. Currently, four FDA-approved HDAC inhibitors are
in use for the treatment of different types of cancers and tumors [16]. Also, suberoylanilide hydroxamic acid
(SAHA), a class I and II HDACi, has been approved as a treatment for cutaneous
T-cell lymphoma [17]. The benzamide-based drug, MGCD 0103
exhibited potent and selective antiproliferative activities against a broad
spectrum of human cancer cell lines in vitro and in vivo [18]. Clinical trials of many of these FDA-approved
HDAC inhibitors, however, failed to prove efficacy and effectiveness due to
lack of complete understanding of their mechanisms at the cellular level. Experimental
evidence suggests that inhibition of HDAC may protect neuronal cells from brain
ischemia injury and other neurodegenerative disorders [19-21]. Nevertheless, their effect at the cellular level during
many neuropathological conditions such as neuronal ischemic injury and micro-vacuolation
of the cerebral cortex needs to be explored.
NT2 cells are differentiated into neuronal cells upon
retinoic acid treatment [22]. These differentiated neuronal cells
express many structural proteins such as microtubule-associated proteins (MAPs)
and neuronal cell adhesion molecules (NCAMs) as well as and functional proteins
such as calmodulin and G-protein-coupled receptors (GPCRs) and thus could be
used to mimic molecular signaling underlying human neurodegenerative diseases
and disorders. Accumulation of the neurotransmitter glutamate around the
neurons increases Ca2+ influx that activates many signaling pathways [23,24]. Effect of HDAC inhibitors in the mitigation of
oxidative stress, inflammation and apoptosis in these hyper-active
differentiated neuronal cells has not been fully elucidated. In our present
study, we investigated the effect of two chemically diverse HDAC inhibitors
(Vorinostat; SAHA and Mocetinostat; MGCD0103) on different cellular functions
in hypoxia-exposed neuronal cells.
2.0 MATERIALS AND METHODS
2.1 Cell
culture and viability assay
Human pluripotent stem cell-derived NTERA‐2
cl.D1 (NT2/D1; ATCC) neuronal precursor cells were cultured in Dulbecco's
modified Eagle's medium supplemented with 10% fetal bovine serum and 1%
penicillin-streptomycin-glutamine at 37°C with 5% CO2. Differentiation
of NT2 precursor cells into neurons was performed following standard protocols [22,25]. NT2 cells were treated with 10 μM retinoic acid
every 4 days for 4 weeks, followed by replating of these cells in poly-D-lysine
(Sigma, India; P8920) coated culture vessels for another 7 days before being
used for experiments. Hypoxic incubation was performed by exposing
differentiated neuronal cells to 300 μM cobalt chloride (CoCl2;
Sigma, India; C8661) for 6 h at 37°C. Following hypoxia, cells were treated
with two chemically different HDAC inhibitors for an additional 18 hours-
Vorinostat (SAHA; Sigma India SML0061; 50μM final concentration) or
Mocetinostat (MGCD0103; Selleckchem India S1122; 1μM final concentration).
Cell viability was determined at indicated time points using the trypan blue
exclusion assay.
2.2 Cellular
ROS assay
1x104 differentiated neuronal cells were
seeded in 96-well tissue culture plates and exposed to normoxic or hypoxic conditions
with chemical inhibitors as described above. Cellular oxidative stress was
determined via measurement of ROS generation. Briefly, at the end of the
experiment at 24 hours, cells were incubated with 25μM 2',7'-dichlorofluorescein
diacetate (DCFDA; Invitrogen D399) for 30 minutes. The fluorescence signal was
measured on a plate reader (Ex484/Em535).
2.3 Enzyme-linked
immunosorbent assay
(ELISA)
96-well plates were coated with 250ng of capture
antibody (HIF-1α, Novus NB100-105) and incubated overnight
at 4oC. Next day, the plates were acclimated at room temperature
(RT) and washed twice with washing buffer (PBS containing 0.1% Tween 20 and 1%
BSA). Non-specific sites were blocked with 5% BSA for 30min at RT. The plates
were washed and incubated for 2h at RT with 100µl of 1:10 dilution of antigen
(1mg/ml of HDAC inhibitor-treated or control protein samples). Unbound antibody
was removed by washing thrice with washing buffer. After washing, 100µl of
1:500 dilution of horseradish peroxidase-conjugated IgG specific secondary
antibody was added to each well. Color was developed by adding 50µl of 0.1%
substrate ABTS [2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)]
(Sigma, A9941) in 0.1M sodium citrate buffer (pH4.5) and 0.1% H2O2
for 15 min at RT in dark. Finally, the reaction was stopped by adding 20ul of
10% SDS to each well. OD was recorded at 492nm in ELISA reader (BioRad, USA).
Graphs were plotted and calculation was done to estimate the concentration of
protein present in the samples using standard recombinant protein plot.
2.4 Quantitative
reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted using Trizol Reagent
(Invitrogen, 15596026) following the manufacturer's instructions. qPCR analysis
was performed using the qScript One-Step RT-qPCR Kit (Quantabio, 95057) and
specific primers (Table 1). The mRNA levels were normalized to β-actin and
expressed as fold change from controls using the 2-ΔΔCt
method.
Table 1. Primer sequences for qRT-PCR analysis
|
Gene target
|
5' primer
|
3' primer
|
|
HMOX-1
|
CAAAGTGCAAGATTCTGCCC
|
CAACTGTCGCCACCAGAAAG
|
|
TAU
|
GTAAAAGCAAAGACGGGACTGG
|
ATGATGGATGTTGCCTAATGAG
|
|
GluR
|
AACCTGCAGAACCGCAAG
|
GCTTGATGAGCAGGTCTATGC
|
|
BAX
|
TGATGGACGGGTCCGGG
|
TCCTGGATGAAACCCTGAAGC
|
|
BCL-xL
|
AGGCGGATTTGAATCTCTTTCTCT
|
GGGCTCAACCAGTCCATTGT
|
|
IL-1β
|
GCCAATCTTCATTGCTCAAGT
|
AGCCATCATTTCACTGGCGA
|
|
β-Actin
|
TCATTCCAAATATGAGATGCG
|
TAGAGAGAAGTGGGGTGGCT
|
2.5 Statistical
analysis
Data were statistically analyzed in GraphPad Prism and
presented as mean±SEM. One-way or two-way ANOVA or t-test was
performed, and p < 0.05 was considered significant. Results are
presented from at least three independent experiments.
3.0 RESULTS
3.1 HDAC
inhibitors mitigate oxidative stress and improve NT2-N survival after hypoxic injury
As demonstrated by several groups, four weeks of
retinoic acid treatment renders NT-2 cells to differentiate into neuronal cells
(NT2-N) (Figure 1A-1C). Hypoxia was induced in these differentiated neuronal
cells by using CoCl2 and at 6h, there was a significant reduction
(30%) in cell survival (Figure 1D). Time-dependent effect of HDAC inhibitors on
CoCl2-treated NT2-N cells indicated dramatic
maintenance in cell survival after 6h of treatment, which was 80 - 90% in SAHA
treated group and 85 - 95% in MGCD0103-treated group compared to hypoxia group
(80 - 85%). When compared to 24h of CoCl2-treated hypoxia condition,
both HDAC inhibitors improved cell survival at an average of 20%. Furthermore,
increased oxidative stress by ROS production in hypoxia condition (2.5 fold
compared to control) was found to be significantly decreased by 40% in both the
inhibitor treatment groups (Figure 1E).

Figure 1. Effect of HDAC inhibitors on hypoxia-induced cell
death, ROS generation, and HIF-1α expression. (A) Schematic for
experimental design of chemically induced hypoxia (+CoCl2) and cell treatments.
(B) Bright-field image of NT2 differentiated neurons, scale bar= 50mm. (C) NT2-N differentiation
markers, TAU and GluR, as assessed by qRT-PCR. *p<0.05
vs. NT2, n=3, mean±SEM. (D) Cell viability, assessed via trypan blue
exclusion assay, at various time points. *p<0.05 vs. normoxia, #p<0.05
vs. hypoxia at respective time points, n=6, mean±SEM.
(E) ROS generation quantification at endpoint (24h). *p<0.05
vs. normoxia, #p<0.05 vs. hypoxia, n=3, mean±SEM; (F) HIF-1α protein expression assessed via ELISA at
endpoint. *p<0.05 vs. normoxia, #p<0.05 vs.
hypoxia, Φp<0.05 as indicated, n=4, mean±SEM.
The classical hypoxia marker, HIF-1α, was elevated by approximately two-fold in CoCl2-treated
NT2-N cells and shown to decrease HIF-1α
expression upon HDAC inhibitor treatment. This suggests that HDAC inhibitors
improve cellular response to hypoxia-induced oxidative stress (Figure 1F). As
compared to SAHA treated group, HIF-1α expression in
MGCD0103-treated hypoxic NT2-N cells was lower with improved cell survival
compared to SAHA, suggesting MGCD0103 may be a potent anti-hypoxic inhibitor.
These inhibitors showed no effect in normoxic cells.
3.2 HDAC
inhibitors promote anti-oxidant response and mitigate heme oxygenase 1- induced
apoptosis and inflammation
Effect of SAHA and MGCD0103 on anti-oxidant HMOX1 (Figure
2A), as well as pro-inflammatory cytokine IL-1β (Figure 2B), in CoCl2-treated
neuronal cells, was assessed by qRT-PCR. There was a
substantial increase (~4-fold) in HMOX1 gene expression in hypoxic NT2-N
cells. Administration of HDAC inhibitors significantly inhibited HMOX1
gene expression by 50%. Similarly, the pro-inflammatory cytokine IL-1β gene expression was reduced to about 50% by these two
HDAC inhibitors in hypoxic neurons. The ratio of pro-apoptotic BAX and
anti-apoptotic BCL-xL genes was determined using qRT-PCR (Figure 2C). As
anticipated, the BAX/BCL-xL gene expression ratio was strongly upregulated
(1.7-fold) in response to hypoxia. Intriguingly, this increase in BAX/BCL-xL
ratio was dramatically reduced to normal levels in the presence of HDAC inhibitors,
SAHA or MGCD0103.
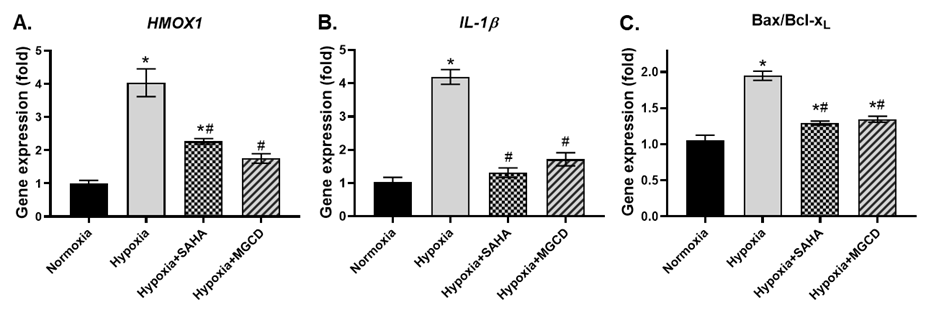
Figure 2. Effect of HDAC inhibitors on hypoxia-induced
expression of oxidative stress, inflammatory and apoptotic gene expression. mRNA
expression of (A) HMOX1; (B) IL-1b; and (C) Bax/Bcl-xL at endpoint. *p<0.05
vs normoxia, #p<0.05 vs hypoxia, n=3-4, mean±SEM.
4.0
DISCUSSION & CONCLUSION
The regulation and modification of non-histone
proteins are crucial to controlling of a multitude of cellular functions.
Histone deacetylases act on these non-histone proteins and alter cellular
signaling through post-translational modification [26]. Modification of transcriptional gene regulation by epigenetic modifiers
may alter OS and reduce cell death [13,15]. Hypoxia-induced OS-mediated stabilization of
HIF-1α is well established [11]. We have shown that inhibitors of
epigenetic modifiers such as mocetinostat can greatly influence HIF-1α expression in chemically induced hypoxia in neuronal
cells via repression in ROS production. Inhibition of HIF-1α was significantly higher in MGCD0103 treated cells
than SAHA, suggesting MGCD0103 modulates HIF-1α
induced hypoxia through ROS inhibition stronger than SAHA. Role of HIF-1α in HO-1 gene regulation was previously established [27], which is regulated through E2-related
factor 2 (Nrf2)/AKT pathway [12]. HMOX1 gene activity in oxidative stress
condition induced by CoCl2 has been shown. Inhibition of HMOX1 by
HDAC inhibitors is essential to inhibit HIF-1α, which is evident through our study. Increased HMOX1 in CoCl2-induced
hypoxic NT2-N cells was mitigated by HDAC inhibitors, suggesting that HDAC
inhibition destabilizes hypoxic factor HIF-1α
and ROS production. Furthermore, HMOX1 regulates many inflammatory responses.
HO-1 is induced by HIF-1α to modulate the activation and function of
different inflammatory cells [28]. We analyzed inflammatory behavior in
these hypoxic neuronal cells in responses to HDAC inhibitors. In CoCl2-induced
hypoxia, increased HMOX1 gene activity post-transcriptionally enhanced
inflammatory gene IL-1β
expression. Furthermore, increased
IL-1β in hypoxic neuronal cells was reduced by
HDAC inhibitors suggesting that HDAC
inhibitors may have anti-inflammatory property. Inflammasome mediated caspase-1 drives maturation of IL-1β. While we did not measure caspase 1 activity, nevertheless
it is known that caspase-1
activation leads to caspase-3 activation and apoptotic cell death [29]. On the other hand, Caspase-2,-3 and -9
are transiently activated, during the RA-induced neuronal differentiation of
NT2 cells [30]. Also, cleavage of caspase-3 induces
apoptosis via mitochondrial pro-apoptotic protein Bax. Increased ratio of Bax
over anti-apoptotic protein Bcl-xL was mitigated by HDAC
inhibitors. Overall, this study suggests that both Vorinostat and Mocetinostat are
potent regulators of ROS-mediated hypoxia in neuronal cells and modulate, resulting
apoptosis and inflammation.
Further studies directed at studying the effect of these epigenetic modulators
using in vivo models of neuroinflammation and neurodegenerative diseases will
provide a rationale for the repurposing of these drugs and others in clinical
trials to treat patients with neuropathological conditions.
Acknowledgements: This work was partly supported by funding from
Pratima Bagchi Memorial Research Society. R.A.B. is supported by a postdoctoral
fellowship award from the Canadian Institutes of Health Research.
Author Contributions: R.A.B. and A.K.B conceived and designed the
experiments; R.A.B., A.K.B. and A.S. performed the experiments; R.A.B., A.K.B.,
A.S., D.K.H and P.H.P. analyzed the data; all authors contributed to the writing
of the manuscript.
Conflicts of Interest: The authors declare no conflict of interest.
References
1. Samanta D, Prabhakar NR, Semenza GL.
Systems biology of oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med.
2017;9(4). https://doi.org/10.1002/wsbm.1382
2. Alarifi S, Ali D, Omar Suliman Y Al, Ahamed
M, Siddiqui MA, Al-Khedhairy AA. Oxidative stress contributes to cobalt oxide
nanoparticles-induced cytotoxicity and DNA damage in human hepatocarcinoma
cells. Int J Nanomedicine. 2013;8(10):189-199. https://doi.org/10.2147/IJN.S37924
3. Uttara B, Singh AV, Zamboni P, Mahajan RT.
Oxidative stress and neurodegenerative diseases: a review of upstream and
downstream antioxidant therapeutic options. Curr Neuropharmacol.
2009;7(1):65-74. https://doi.org/10.2174/157015909787602823
4. Unal-Cevik I, Kilinç M, Can A,
Gürsoy-Ozdemir Y, Dalkara T. Apoptotic and necrotic death mechanisms are
concomitantly activated in the same cell after cerebral ischemia. Stroke.
2004;35(9):2189-2194. https://doi.org/10.1161/01.STR.0000136149.81831.c5
5. DeGracia DJ, Kumar R, Owen CR, Krause GS,
White BC. Molecular pathways of protein synthesis inhibition during brain
reperfusion: implications for neuronal survival or death. J Cereb Blood Flow
Metab. 2002;22(2):127-141. https://doi.org/10.1097/00004647-200202000-00001
6. Turrens JF. Mitochondrial formation of
reactive oxygen species. J Physiol (Lond). 2003;552(Pt 2):335-344. https://doi.org/10.1113/jphysiol.2003.049478
7. Adam-Vizi V. Production of reactive oxygen
species in brain mitochondria: contribution by electron transport chain and
non-electron transport chain sources. Antioxid Redox Signal. 2005;7(9-10):1140-1149.
https://doi.org/10.1089/ars.2005.7.1140
8. Wang GL, Jiang BH, Rue EA, Semenza GL.
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer
regulated by cellular O2 tension. Proc Natl Acad Sci USA.
1995;92(12):5510-5514. https://doi.org/10.1073/pnas.92.12.5510
9. Semenza GL. Hypoxia-inducible factor 1 and
cardiovascular disease. Annu Rev Physiol. 2013;76:39-56. https://doi.org/10.1146/annurev-physiol-021113-170322
10. Poss KD, Tonegawa S. Reduced stress defense
in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA.
1997;94(20):10925-10930. https://doi.org/10.1073/pnas.94.20.10925
11. Movafagh S, Crook S, Vo K. Regulation of
hypoxia-inducible factor-1a by reactive oxygen species: new developments in an
old debate. J Cell Biochem. 2014;116(5):696-703. https://doi.org/10.1002/jcb.25074
12. Zhao R, Feng J, He G. Hypoxia increases
Nrf2-induced HO-1 expression via the PI3K/Akt pathway. Front Biosci
(Landmark Ed). 2016;21:385-396. https://www.ncbi.nlm.nih.gov/pubmed/26709780
13. Schweizer S, Meisel A, Märschenz S.
Epigenetic mechanisms in cerebral ischemia. J Cereb Blood Flow Metab.
2013;33(9):1335-1346. https://doi.org/10.1038/jcbfm.2013.93
14. Semenza GL. Transcriptional regulation by
hypoxia-inducible factor 1 molecular mechanisms of oxygen homeostasis. Trends
Cardiovasc Med. 1996;6(5):151-157. https://doi.org/10.1016/1050-1738(96)00039-4
15. Shimazu T, Hirschey MD, Newman J, He W,
Shirakawa K, Le Moan N, et al. Suppression of oxidative stress by
β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science.
2012;339(6116):211-214. https://doi.org/10.1126/science.1227166
16. Hull EE, Montgomery MR, Leyva KJ. HDAC
Inhibitors as Epigenetic Regulators of the Immune System: Impacts on Cancer
Therapy and Inflammatory Diseases. BioMed Research International.
2016;2016:8797206. https://doi.org/10.1155/2016/8797206
17. Rangwala S, Zhang C, Duvic M. HDAC
inhibitors for the treatment of cutaneous T-cell lymphomas. Future Med Chem.
2012;4(4):471-486. https://doi.org/10.4155/fmc.12.6
18. Fournel M, Bonfils C, Hou Y, Yan PT,
Trachy-Bourget M-C, Kalita A, et al. MGCD0103, a novel isotype-selective
histone deacetylase inhibitor, has broad spectrum antitumor activity in vitro
and in vivo. Mol Cancer Ther. 2008;7(4):759-768. https://doi.org/10.1158/1535-7163.MCT-07-2026
19. Morrison BE, Majdzadeh N, Zhang X, Lyles A,
Bassel-Duby R, Olson EN, et al. Neuroprotection by histone deacetylase-related
protein. Molecular and Cellular Biology. 2006;26(9):3550-3564. https://doi.org/10.1128/MCB.26.9.3550-3564.2006
20. Butler KV, Kalin J, Brochier C, Vistoli G,
Langley B, Kozikowski AP. Rational design and simple chemistry yield a
superior, neuroprotective HDAC6 inhibitor, tubastatin A. J Am Chem Soc.
2010;132(31):10842-10846. https://doi.org/10.1021/ja102758v
21. Ziemka-Nalecz M, Zalewska T.
Neuroprotective effects of histone deacetylase inhibitors in brain ischemia. Acta
Neurobiol Exp (Wars). 2015;74(4):383-395. https://www.ncbi.nlm.nih.gov/pubmed/25576969
22. Langlois A, Duval D. Differentiation of the
human NT2 cells into neurons and glia. Methods Cell Sci. 1997;19:213-219.
https://doi.org/10.1023/A:1009731707443
23. Younkin DP, Tang CM, Hardy M, Reddy UR, Shi
QY, Pleasure SJ, et al. Inducible expression of neuronal glutamate receptor
channels in the NT2 human cell line. Proc Natl Acad Sci USA.
1993;90(6):2174-2178. https://doi.org/10.1073/pnas.90.6.2174
24. Hardy M, Younkin D, Tang CM, Pleasure J,
Shi QY, Williams M, et al. Expression of non-NMDA glutamate receptor channel
genes by clonal human neurons. J Neurochem. 1994;63(2):482-489. https://doi.org/10.1046/j.1471-4159.1994.63020482.x
25. Haile Y, Fu W, Shi B, Westaway D, Baker G,
Jhamandas J, et al. Characterization of the NT2-derived neuronal and astrocytic
cell lines as alternative in vitro models for primary human neurons and
astrocytes. J Neurosci Res. 2014;92(9):1187-1198. https://doi.org/10.1002/jnr.23399
26. Zhang X, Yuan Z, Zhang Y, Yong S,
Salas-Burgos A, Koomen J, et al. HDAC6 modulates cell motility by altering the
acetylation level of cortactin. Neuroimage. 2007;27(2):197-213. https://doi.org/10.1016/j.molcel.2007.05.033
27. Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J,
Semenza GL, et al. Hypoxia-inducible factor-1 mediates transcriptional
activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem.
1997;272(9):5375-5381. https://doi.org/10.33860/jbc.v1i1.186
28. Sebastián VP, Salazar GA, Coronado-Arrázola
I, Schultz BM, Vallejos OP, Berkowitz L, et al. Heme Oxygenase-1 as a Modulator
of Intestinal Inflammation Development and Progression. Front Immunol.
2018;9:1956. https://doi.org/10.3389/fimmu.2018.01956
29. Sagulenko V, Vitak N, Vajjhala PR, Vince
JE, Stacey KJ. Caspase-1 Is an Apical Caspase Leading to Caspase-3 Cleavage in
the AIM2 Inflammasome Response, Independent of Caspase-8. J Mol Biol.
2017;430(2):238-247. https://doi.org/10.1016/j.jmb.2017.10.028
30. Pistritto G, Papaleo
V, Sanchez P, Ceci C, Barbaccia ML. Divergent modulation of neuronal differentiation
by caspase-2 and -9. PLoS ONE. 2012;7(5):e36002. https://doi.org/10.1371/journal.pone.0036002
![]()

