Ultrastructural
study of sciatic nerve in Ts1Cje mouse model for Down syndrome: an implication
of peripheral nerve defects in hypotonia
Usman Bala 1,2,3, Melody Pui-Yee
Leong 2,4, Chai Ling Lim 1,2, Hayati Kadir Shahar 5,
Othman Fauziah 1, Mei-I Lai 2,6, King-Hwa Ling 2,3
and Pike-See Cheah 1,2,*
1 Department of Human Anatomy, Faculty of
Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Selangor,
Malaysia.
2 Genetics and Regenerative Medicine
Research Centre (GRMRC), Faculty of Medicine and Health Sciences, Universiti
Putra Malaysia, Serdang, Selangor, Malaysia.
3 Department of Human Anatomy, College of
Medical Sciences, Gombe State University, Gombe, Nigeria.
4 Department of Biomedical Sciences, Faculty
of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Selangor,
Malaysia.
5 Department of Community Health, Faculty
of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Selangor,
Malaysia.
6 Department of Pathology, Faculty of
Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Selangor,
Malaysia.
* Correspondence: cheahpikesee@upm.edu.my; Tel.:
+603-8947 2355
Received: 16 August 2018; Accepted: 25 September 2018; Published: 16
October 2018
Edited by: Zurina Hassan (Universiti Sains Malaysia)
Reviewed by: Mohd. Farooq Shaikh (Monash University Malaysia);
Wael MY Mohamed (International Islamic University
Malaysia)
https://doi.org/10.31117/neuroscirn.v1i2.17
Abstract: Trisomy 21 is chromosomal
abnormality that occurs as a result of triplication of human chromosome 21
(Hsa21), a condition also known as Down syndrome (DS). Beside the intellectual
disability and systems anomalies, motor dysfunction due to hypotonia has also
been characterised in individuals with DS and yet, its etiology remains
unclear. Ts1Cje, a mouse model for DS, has a partial trisomy (Mmu16)
homology to Hsa21, is widely used for DS research. This study investigated the
morphological changes and degree of myelination in sciatic nerves of the Ts1Cje
mice using both light and transmission electron microscopes processed images.
The result showed no morphological difference in the sciatic nerve between the
Ts1Cje and WT mice. The g ratio of the Ts1Cje mice was significantly (P<0.0001)
higher compared to that of the WT mice. Two factors are known to determine the
g ratio, the axonal diameter and the myelin thickness. There was no significant
(P=0.2146) difference in the axonal diameter between the two genotypes.
Interestingly, the myelin thickness was significantly (P<0.0001)
thinner in nerve fibres of the Ts1Cje mice as compared to that of the WT mice.
It is therefore concluded that, the hypomyelination in Ts1Cje mice may affect
the conduction velocity which in turn affect their motor activity.
Keywords: Down syndrome; hypotonia; Ts1Cje mice; sciatic nerve;
muscle weakness;
©2018 by Bala et al for use and distribution in accord with the
Creative Commons Attribution (CC BY-NC 4.0) license (https://creativecommons.org/licenses/by-nc/4.0/),
which permits unrestricted non-commercial use, distribution, and reproduction
in any medium, provided the original author and source are credited.
1. INTRODUCTION
Trisomy 21 is chromosomal abnormality that occurs as a
result of triplication of human chromosome 21 (Hsa21), a condition also known
as Down syndrome (DS) [1]. The most common cause of DS is
chromosomal non-disjunction [2] and it is associated with factors such as
genetics, increased maternal age and low socio-economic status [2,3]. Beside the intellectual disability and systems
anomalies, motor dysfunction due to hypotonia has also been characterised in
individuals with DS [4]. Delayed in motor function development,
difficulties in motor planning and instabilities in trunk and postural control [5] together with joints stiffness[6] seen in individuals with DS have
significantly affect their ability to perform some functional tasks [7] thus resulting great limitations in labour
productivity and socio-economic development [8]. Evidence based studies have indicated the
involvement of the central nervous system (CNS) disorder [9] and the defective neuromuscular junction [10] as likely factors in manifestation of
hypotonia in individuals with DS. However peripheral nerve defects cannot be
simply ruled out.
Over years, different mouse models of DS have been
generated and each model presents similar phenotypes seen in individuals with
DS [11]. Several motor dysfunctions, such as
delayed motor skill development, poor fine motor movement and impaired motor
coordination have been reported in some of these mouse models [12]. The Hsa21 shares a conserved homology
with 3 regions of mouse chromosomes, Mmu10, Mmu16, and Mmu 17,
and each chromosome contains a segment of genes that are trisomic to a
different region of Hsa21 [13].
The Ts1Cje is a mouse model for DS that carries a
shorter region of Mmu16 with approximately 85 genes that are homologous
to genes located on Hsa21 [14]. Over years, studies showed that the
Ts1Cje exhibits features associated with DS such as brain abnormality [15]. Recent studies have indicated that, this
mouse model for DS exhibits motor dysfunction with weak muscle strength, poor
balance and impaired motor coordination [16]. In addition, decreased in the population
of type I muscle fibre and reduced nerve conduction velocity have been observed
in this mouse model [16]. Yet, the possible factors contributed to
reduced nerve conduction velocity in Ts1Cje mouse model for DS has not been
reported.
The sciatic nerve being the largest peripheral nerve
in the body, is also the most preferred and widely chosen nerve in most neurological
research and peripheral nerve-related studies [17]. This nerve was chosen in preference to
the other nerves in this study mainly due to its size, location and functional
diversity.
Optimal theoretical G-ratio of 0.6 is reported for a
myelinated axon and it is well accepted that this ratio is optimised to achieve
maximal efficiency and physiological optimization [18]. The present study aims to investigate the
possible contribution of sciatic nerve defects in relation to muscle weakness
in Ts1Cje mice. To understand the possible contribution of morphological
changes and degree of myelination in the peripheral nerve to muscle weakness
seen in individuals with DS, ultrastructural and light microscopic studies of
the sciatic nerves of the Ts1Cje mice were performed. Both the morphological
changes and degree of myelination in the sciatic nerve of Ts1Cje mouse model
for DS were also assessed.
2. MATERIALS AND METHODS
2.1 Ethical approval, animal breeding, genotyping and animal
husbandry
Institutional
Animal Care and Use Committee (IACUC), Faculty of Medicine and Health Sciences,
Universiti Putra Malaysia (UPM) approved this study (Reference number:
UPM/FPSK/PADS/BR-UUH/00494). The animals were handled in accordance with IACUC
guidelines. Two groups of
mice, the Ts1Cje male (n=8) and wildtype (WT) (C57BL/6) male (n=8) at postnatal
day 60-70 were used. The mice were generated by mating Ts1Cje of
C57BL/6 background males (obtained from Walter and Eliza Hall Institute for
Medical Research, Australia) with WT C57BL/6 females. The Ts1Cje littermates were identified by polymerase chain reaction (PCR) genotyping
from tail biopsy genomic DNA that amplified the Grik1 gene in WT mice,
while both Grik1 and Neo genes in the Ts1Cje mouse as described [14]. The
disomic littermates with the WT genotype served as the normal control. All mice were housed under controlled temperatures
with a 12:12 hour light-dark cycle in the mouse room facility, Genetics and Regenerative Medicine Research
Centre (GRMRC), UPM. The mice were given
unlimited access to standard animal feed (Altromin 1324, Germany) and clean
water ad libitum.
2.2 Sample collection
The mice were deeply anaesthetised with inhaled 4%
(v/v) isoflurane prior to cervical dislocation to eliminate perception of pain.
The sciatic nerves were harvested as described [17]. The harvested nerves (both sides) were
washed with chilled 1X phosphate buffered saline (PBS), cut into small pieces
(1mm2) and fixed in 4% glutaraldehyde at room temperature for 24
hours.
2.3 Ultra-structural assessment of the sciatic nerve
Tissue blocks of
the sciatic nerve were prepared according to the standard protocol for
ultrathin sections as described[18]. A semithin coronal section (1 μm) of
the sciatic nerve was obtained using a diamond knife (Leica, 4mm, 45°), and
stained with toluidine blue at ~70°C for 10 seconds. The sections were washed
with distilled water and dried on hot plate (Thermolyne Nuova HP). Ultrathin
coronal sections (70 nm) of the sciatic nerve were collected and stained with
uranyl acetate and Reynolds lead citrate. Briefly, the sections were
submerged in a few drops of the uranyl acetate for 20 minutes at 40°C followed
by stream-washed in distilled water for 15 seconds. Then, sections were
submerged in Reynolds lead citrate surrounded by square-arranged NaOH for 10
minutes, stream-washed with distilled water and allowed to dry.
Ultra-structural images were obtained using transmission electron microscope
(TEM) (HITACHI H-7100). Ultrastructures of the sciatic nerves such as the
lamellae, mitochondria, cytoskeletal (neurofilaments and microtubules) were
evaluated.
2.4 Light
microscopic assessment and G ratio measurement of the sciatic nerve
A semithin sections (1 μm) were examined using a
light microscope (Olympus BX51, Japan) and images were captured at 100X
magnification through CCD digital camera (Olympus DP72, Japan).
Subsequently, morphological analysis of the acquired images was carried out
using Image J software (National Institutes of Health, Bethesda, MD) plug-in (g
ratio calculator). The original programme was downloaded from http://cifweb.unil.ch of Cellular Imaging Facility (CIF) of the
University of Lausanne. G ratio analysis was performed according to the G ratio
Calculator 1.0 manual of CIF (Version 1.0, dated 01/03/2007). Briefly,
micrograph images were pre-processed by converting them into 8-bit grayscale
format and background noise was removed using a median filter for better edge
detection. A user define threshold (100 to 110) was applied to binarize the
images. Three slides were selected from each sample (n=8 per group) and four
images (from each quadrant) were obtained from each slide for measurement and
analysis. All myelinated nerve fibres on each image were selected and
measured. Unmyelinated and smaller (developing nerve fibres) nerve fibres were
excluded from measurement. Approximately 940 to 975 nerve fibres were randomly
selected from each group for morphometric measurement. All morphological
measurement and analysis were performed based on single-blinded approach with
respect to the mouse genotype. The g ratio was calculated based on the ratio of the inner axon (d1) to outer axon diameter (d2).
2.5 Statistical analysis
G ratio was computed and analysed using Prism7
statistical software (GraphPad Software, USA). Student's t-test was used
to compare the numerical values obtained from the two groups/genotypes. The
results were expressed as mean±SEM and the probability values of P
< 0.05 were considered statistically significant.
3. RESULTS
3.1
Ultrastructural morphology of the sciatic nerve
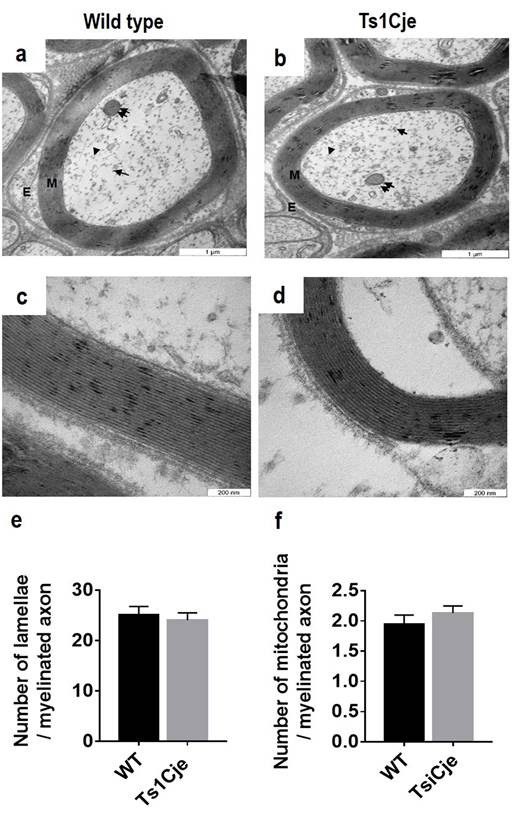
An ultrastructural analysis of the sciatic nerve
fibres revealed no morphological differences between the WT (Figure 1a) and the
Ts1Cje mice (Figure 1b). No axonal alteration either in the form of myelin
sheath distortion or degeneration in the Ts1Cje mice. The myelin lamellae were
well preserved in both genotypes [WT (Figure 1c); Ts1Cje (Figure 1d)]. The
number of lamellae of individual myelinated axon was further quantified and the
result showed no significant differences in the average of the lamellae between
the Ts1Cje mice (24.06±1.45, n=24) compared to that of the WT mice (25.09±1.70,
n=30, t=0.60, P=0.46) (Figure 1e). In addition, cytoskeletal
structures such as neurofilaments and microtubules were homogeneously
distributed in the nerve fibres of the Ts1Cje mice, similar to WT (data not
shown). The morphology, distribution and population of the mitochondria were
evaluated in the nerve fibres. There was no morphological anomaly or
aggregation of mitochondria in the nerve fibres of Ts1Cje mice. Similarly,
there was no significant differences in the average number of the mitochondria
in the myelinated fibres between the two genotypes [(WT=1.94±0.15, n=54);
(Ts1Cje=2.13±0.12, n=77, t=0.9621, P=0.3381)] (Figure 1f).
3.2 G ratio
analysis of the sciatic nerve
To investigate the potential role of the peripheral
nerve defects in contributing to muscle weakness in DS, morphometric analysis
of the sciatic nerve fibres between the two genotypes was performed using light
microscopic images (Figure 2a). The g ratio was computed using g ratio
calculator and the result was compared between the Ts1Cje and WT mice. According
to the previous study, the optimized value of G-ratio ranged 0.55-0.68 [18]. The g ratio of the Ts1Cje mice (0.714±0.002, n=974) was significantly higher compared to that of the WT mice (0.656±0.002, n=944, t=19.46, P<0.0001) (Figure 2b). There are two
major factors that affect the g ratio value; myelin thickness and the size of
the axon. Further analysis was performed to evaluate the axonal diameter of the
Ts1Cje and WT mice. The result showed no significant difference between the
axonal diameter of the Ts1Cje (range=1.08-3.31; 1.895±0.011μm, n=975) and WT
mice (range=0.87-3.27; 1.873±0.013μm, n=943; t=1.335, P=0.1821)
(Figure 2c). Subsequent analysis indicated that the myelin thickness was
significantly thinner in nerve fibres of the Ts1Cje mice (range=0.08-0.97;
0.372±0.003 μm n=971) as compared to that of the WT mice (range=0.21 - 1.95;
0.487±0.004 μm n=943; t=24.81; P<0.0001) (Figure 2d).
Scatter plots (Figures 2e-g) show the pattern of distribution of axonal
diameter and myelin sheath thickness. The distribution of fibres shifted to the
left in the plots of Ts1Cje and this indicates high population of the fibres
with thinner myelin sheaths as compared to the WT mice.
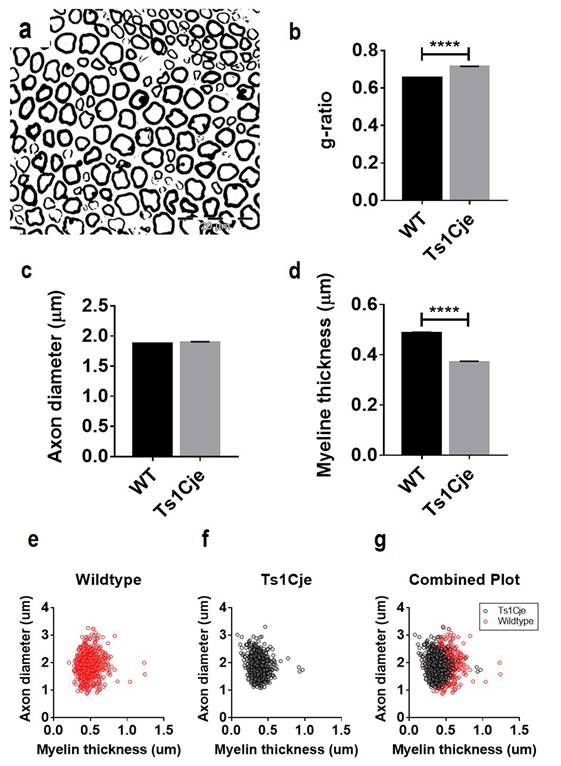
Figure 2.
Evaluation of g ratio and myelin thickness in the Ts1Cje mice. (a) Semithin
sections of the sciatic nerves were used for g ratio calculation. (b) Morphometric
analysis of the nerve fibres revealed a significantly (P<0.0001) higher g ratio in the Ts1Cje
mice as compared to the WT mice. (c) The axon diameter of the Ts1Cje
mice showed no significant difference (P=0.1821) from that of the WT
mice. (d) Myelin sheath in WT mice was significantly thicker (P<0.0001)
compared to the Ts1Cje mice. (e-g) Scatter plots show the nerve fibres
of both genotypes, confirming the presence of nerve fibres of Ts1Cje mice have
thinner myelin sheath than that of the WT mice.
4. DISCUSSION
The functional and structural index for assessing
axonal myelination is the ratio of the axon diameter to the myelinated fibre
diameter (g ratio) and the concept of g ratio has been used in assessing the
degree of myelination of the peripheral nerves [19,20]. Recent studies have shown that, the g ratio concept
is useful in evaluating the level of myelination of the sciatic nerve during
aging [21].
The axonal diameter and myelin thickness are two
determinant factors for the g ratio. There is marked association between the myelin
thickness and the axon diameter during nerve development [22]. The axonal diameter significantly
influences the conduction velocity of a nerve fibres [23,24]. In myelinated fibres, changes in the size of the
axon is proportional to the conduction velocity. Thus, an increase in the
axonal diameter results in an increase in the conduction velocity of the nerve
fibre. Nerve fibres with smaller axon diameter are slow in conduction [24]. A recent studies revealed that, Ts1Cje
mice have low conduction velocity as compared with the WT [16]. In this study, the axonal diameter is
relatively the same in both genotypes. Therefore, based on this finding, the
possibility for the axonal diameter to influences the function of the nerve
fibre in Ts1Cje mice may be excluded.
The myelin thickness is inversely proportional to the
g ratio and plays a significant role in conduction velocity. The myelin sheath
in Ts1Cje mice is thinner as compared to their aged matched WT. The low
conduction velocity observed in the adult Ts1Cje mice [16] further support our finding of low myelin
sheath in Ts1Cje mice. It is known that, myelin thickness is proportional to
the conduction velocity and several studies have shown that, hypomyelination
significantly affects the conduction velocity in both human diseased condition [25] and mouse model of peripheral neuropathies
[26]. Thus nerve fibre with a thick myelin
sheath conducts impulse faster than less myelinated or unmyelinated nerve fibre
[23] . The Ts1Cje mice have an intact axonal
diameter but its myelin sheath was significantly thinner as compared to WT
mice. It is therefore tempted to suggest that myelin is the key element
responsible for the low conduction velocity in the adult Ts1Cje mice [16]. However, it may be interesting to
investigate the quality of the connection between the nerve fibres and the
target organ (muscle) at the neuromuscular junction. Axon extends distally to
reach the appropriate target organ during nerve development. When there is an
inappropriate connection between the sprouting axon with the target organ, it
tends to inhibit the supply of necessary growth factors, thus resulting in
defective nerve function. Similarly, to fully understand the factors that may
be responsible for the low conduction in Ts1Cje mice, it is proposed that a
couple of genes contributed in myelin development and/or maintenance such as early
growth response gene 2 (Egr2) [27], myelin protein zero (MPZ) and peripheral
myelin protein 22 (PMP22) [28]. Analysis of peripheral nerve genes
expression profile warrant further investigation to identify the causative
genes involved in defective peripheral nerve myelination of Ts1Cje.
5. CONCLUSIONS
The myelin sheath was significantly thinner in sciatic
nerve of the Ts1Cje mice and this may affect the conduction velocity of the
nerve fibres. Thus, hypomyelination in peripheral nerve of the Ts1Cje may
explain the low conduction velocity and poor motor activity of the Ts1Cje mice.
Acknowledgements: This work was
supported in part by funding from the MOHE Fundamental Research Grant Scheme
(04-01-15-1663FR), UPM Geran Putra IPT (UPM/700/2/1/GP-IPT/2013/9409500) and
UPM Geran Putra IPS (UPM/700/2/1/GP-IPS/2014/9448800) awarded to PSC; UPM
Research University Grant Scheme (04-02-12-2102RU) and MOSTI ScienceFund
(02-01-04-SF2336) awarded to KHL. MPYL and CLL were recipients of the Malaysian
Ministry of Higher Education MyMaster scholarship.
Author Contributions: UB PSC and KHL, designed the experiments. UB, MPYL
and CCL performed the experiments. UB, HKS, KHL and PSC analysed the data. OF,
MIL co-supervised the experiment. UB, KHL and PSC drafted the manuscript.
Conflicts of Interest: The authors declare no conflict of interest.
References
1.
Antonarakis SE, Lyle R, Dermitzakis ET,
Reymond A, Deutsch S. Chromosome 21 and down syndrome: from genomics to
pathophysiology. Nat Rev Genet. 2004;5(10):725-738. https://doi.org/10.1038/nrg1448
2.
Sherman SL, Freeman SB,
Allen EG, Lamb NE. Risk factors for nondisjunction of trisomy 21. Cytogenet
Genome Res. 2005;111(3-4):273-280. https://doi.org/10.1159/000086900
3.
Hunter JE, Allen EG,
Shin M, Bean LJH, Correa A, Druschel C, et al. The association of low
socioeconomic status and the risk of having a child with Down syndrome: a
report from the National Down Syndrome Project. Genet Med.
2013;15(9):698-705. https://doi.org/10.1038/gim.2013.34
4.
Lana-Elola E,
Watson-Scales SD, Fisher EMC, Tybulewicz VLJ. Down syndrome: searching for the
genetic culprits. Dis Model Mech. 2011;4(5):586-595. https://doi.org/10.1242/dmm.008078
5.
Malak R, Kotwicka M,
Krawczyk-Wasielewska A, Mojs E, Samborski W. Motor skills, cognitive
development and balance functions of children with Down syndrome. Ann Agric
Environ Med. 2013;20(4):803-806. https://www.ncbi.nlm.nih.gov/pubmed/24364457
6.
Galli M, Rigoldi C,
Brunner R, Virji-Babul N, Giorgio A. Joint stiffness and gait pattern
evaluation in children with Down syndrome. Gait Posture. 2008;28(3):502-506.
https://doi.org/10.1016/j.gaitpost.2008.03.001
7.
Kasari C, Freeman SF.
Task-related social behavior in children with Down syndrome. Am J Ment
Retard. 2001;106(3):253-264. https://doi.org/10.1352/0895-8017(2001)1062.0.CO;2
8.
Buckley S, Bird G,
Sacks B. Social development for individuals with Down syndrome-An overview.
Down Syndrome Education. 2002; pp. 1-44, ISBN: 9781903806210.
9.
Anderson JS, Nielsen
JA, Ferguson MA, Burback MC, Cox ET, Dai L, et al. Abnormal brain synchrony in
Down Syndrome. Neuroimage Clin. 2013;2:703-715. https://doi.org/10.1016/j.nicl.2013.05.006
10.
Chang KT, Min K-T.
Upregulation of three Drosophila homologs of human chromosome 21 genes alters
synaptic function: implications for Down syndrome. Proc Natl Acad Sci USA.
2009;106(40):17117-17122. https://doi.org/10.1073/pnas.0904397106
11.
Davisson MT, Schmidt C,
Akeson EC. Segmental trisomy of murine chromosome 16: a new model system for
studying Down syndrome. Prog Clin Biol Res. 1990;360:263-280. https://www.ncbi.nlm.nih.gov/pubmed/2147289
12.
Galante M, Jani H,
Vanes L, Daniel H, Fisher EMC, Tybulewicz VLJ, et al. Impairments in motor
coordination without major changes in cerebellar plasticity in the Tc1 mouse
model of Down syndrome. Hum Mol Genet. 2009;18(8):1449-1463. https://doi.org/10.1093/hmg/ddp055
13.
Galdzicki Z, Siarey R,
Pearce R, Stoll J, Rapoport SI. On the cause of mental retardation in Down
syndrome: extrapolation from full and segmental trisomy 16 mouse models. Brain
Res Brain Res Rev. 2001;35(2):115-145. https://doi.org/10.1016/S0926-6410(00)00074-4
14.
Sago H, Carlson EJ,
Smith DJ, Kilbridge J, Rubin EM, Mobley WC, et al. Ts1Cje, a partial trisomy 16
mouse model for Down syndrome, exhibits learning and behavioral abnormalities. Proc
Natl Acad Sci USA. 1998;95(11):6256-6261. https://doi.org/10.1073/pnas.95.11.6256
15.
Olson LE, Roper RJ,
Baxter LL, Carlson EJ, Epstein CJ, Reeves RH. Down syndrome mouse models
Ts65Dn, Ts1Cje, and Ms1Cje/Ts65Dn exhibit variable severity of cerebellar
phenotypes. Dev Dyn. 2004;230(3):581-589. https://doi.org/10.1002/dvdy.20079
16.
Bala U, Leong MP-Y, Lim
CL, Shahar HK, Othman F, Lai M-I, et al. Defects in nerve conduction velocity
and different muscle fibre-type specificity contribute to muscle weakness in
Ts1Cje Down syndrome mouse model. PLoS ONE. 2018;13(5):e0197711. https://doi.org/10.1371/journal.pone.0197711
17.
Bala U, Tan K-L, Ling
KH, Cheah P-S. Harvesting the maximum length of sciatic nerve from adult mice:
a step-by-step approach. BMC Res Notes. 2014;7(1):714. https://doi.org/10.1186/1756-0500-7-714
18.
Chomiak T. What is the
optimal value of the g-ratio for myelinated fibers in the rat CNS? A
theoretical approach. PLoS ONE. 2009;4(11):e7754. https://doi.org/10.1371/journal.pone.0007754
19.
Maturana LG, Pierucci
A, Simões GF, Vidigal M, Duek EAR, Vidal BC, et al. Enhanced peripheral nerve
regeneration by the combination of a polycaprolactone tubular prosthesis and a
scaffold of collagen with supramolecular organization. Brain Behav.
2013;3(4):417-430. https://doi.org/10.1002/brb3.145
20.
Kato N, Matsumoto M,
Kogawa M, Atkins GJ, Findlay DM, Fujikawa T, et al. Critical role of p38 MAPK for
regeneration of the sciatic nerve following crush injury in vivo. J
Neuroinflammation. 2013;10:1. https://doi.org/10.1186/1742-2094-10-1
21.
Ugrenović S,
Jovanović I, Vasović L, Kundalić B, Čukuranović R,
Stefanović V. Morphometric analysis of the diameter and g-ratio of the
myelinated nerve fibers of the human sciatic nerve during the aging process. Anat
Sci Int. 2015;91(3):238-245. https://doi.org/10.1007/s12565-015-0287-9
22.
Kaplan S, Odaci E, Unal
B, Sahin B, Fornaro M. Chapter 2: Development of the peripheral nerve. Int
Rev Neurobiol. 2009;87:9-26. https://doi.org/10.1016/S0074-7742(09)87002-5
23.
Wu LMN, Williams A,
Delaney A, Sherman DL, Brophy PJ. Increasing internodal distance in myelinated
nerves accelerates nerve conduction to a flat maximum. Curr Biol.
2012;22(20):1957-1961. https://doi.org/10.1016/j.cub.2012.08.025
24.
Ikeda M, Oka Y. The
relationship between nerve conduction velocity and fiber morphology during
peripheral nerve regeneration. Brain Behav. 2012;2(4):382-390. https://doi.org/10.1002/brb3.61
25.
Verhoeven K, De Jonghe
P, Van de Putte T, Nelis E, Zwijsen A, Verpoorten N, et al. Slowed conduction
and thin myelination of peripheral nerves associated with mutant rho Guanine-nucleotide
exchange factor 10. Am J Hum Genet. 2003;73(4):926-932. https://doi.org/10.1086/378159
26.
Rünker AE, Kobsar I,
Fink T, Loers G, Tilling T, Putthoff P, et al. Pathology of a mouse mutation in
peripheral myelin protein P0 is characteristic of a severe and early onset form
of human Charcot-Marie-Tooth type 1B disorder. The Journal of Cell Biology.
2004;165(4):565-573. https://doi.org/10.1083/jcb.200402087
27.
Le N, Nagarajan R, Wang
JYT, Araki T, Schmidt RE, Milbrandt J. Analysis of congenital hypomyelinating
Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell
differentiation and myelination. Proc Natl Acad Sci USA.
2005;102(7):2596-2601. https://doi.org/10.1073/pnas.0407836102
28. Nave K-A, Sereda MW, Ehrenreich H.
Mechanisms of disease: inherited demyelinating neuropathies--from basic to
clinical research. Nat Clin Pract Neurol. 2007;3(8):453-464. https://doi.org/10.1038/ncpneuro0583
![]()